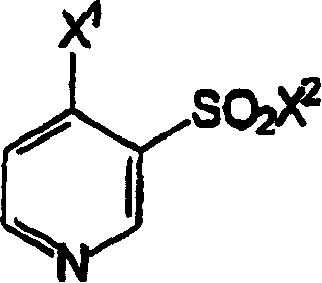
Novel processes for preparing torsemide intermediate
A technology of compound and general formula, which is applied in the new preparation of torasemide intermediate pyridine and the new preparation field of torasemide, which can solve the problem of unsuitable for large-scale manufacturing process, low yield and high yield of torasemide low level problem
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
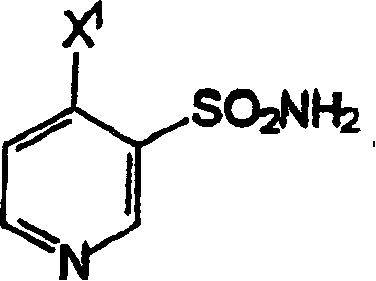
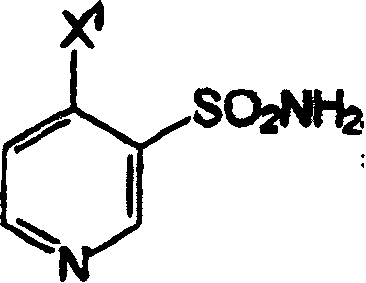
[0058] Synthesis of (3-sulfamoyl-4-chloro)pyridine
[0059] In a 100-ml three-necked flask equipped with a magnetic stirrer, condenser, thermometer, and dropping funnel, (3-sulfonylchloro-4-chloro)pyridine (10 g, 1 equiv, 46.7 mmol) was suspended in MTBE at room temperature (30ml). 25% ammonium hydroxide solution (13.5 ml, 2.13 eq.) was added dropwise to the suspension at such a rate that the temperature rose to about 22°C to about 26°C and the temperature was maintained until all the ammonium hydroxide was added. The suspension was then cooled to room temperature and stirred for 1 hour. The pH of the suspension was adjusted to 8±0.1 by adding a few drops of 25% ammonium hydroxide solution. The suspension was filtered and washed with water (2 x 10ml) and the wet product (-8g) was dried at 40°C under 1 mm Hg vacuum. 6.7 g of (3-sulfamoyl-4-chloro)pyridine were isolated in a yield of 74.4%.
Embodiment 2
[0061] Synthesis of torasemide
[0062] Add acetonitrile (15ml), 3-sulfamoyl-4-(3'-methylphenyl)aminopyridine (5g) and triethylamine ( TEA) (5.3ml). 1.87 ml of isopropyl isocyanate was added dropwise within 10 minutes, and the whole mixture was stirred at 40±2°C to dissolve completely. The mixture was cooled to room temperature and stirred for another 2 hours. The pH of the mixture was adjusted to 4.3 while raising the temperature to about 35°C. The mixture was recooled to room temperature, filtered and rinsed with a mixture of acetonitrile:water (1:1) (10 ml). The wet crude product was triturated in acetonitrile:water mixture (5:1, 13ml) at 60°C for half an hour, filtered and rinsed with acetonitrile:water (5:1) mixture (2x7ml). The milled product was then dried at 50° C. under high vacuum (3 mmHg) for 6 hours to yield 5.4 g of crude torasemide (81.5% crude yield).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com