Asymmetric synthesis of benzoxazinones
A compound and asymmetric technology, applied in the direction of drug combination, organic chemistry, organic active ingredients, etc., can solve instability, expensive and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
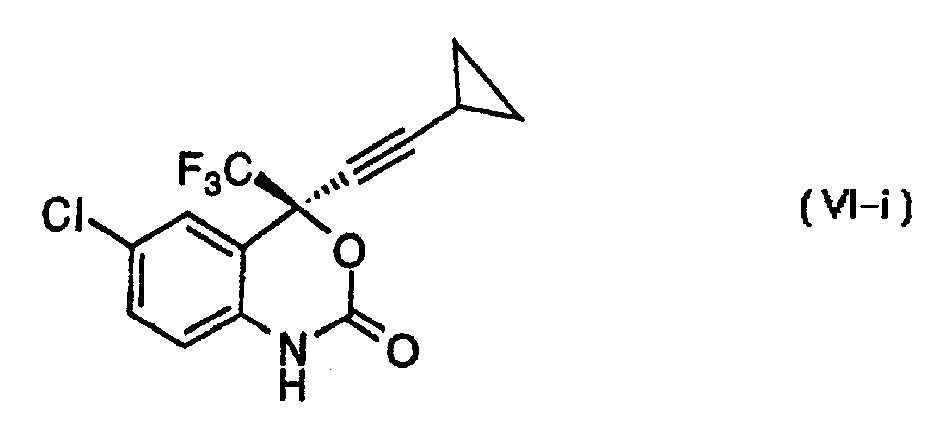
[0031] In a preferred embodiment, the compound of formula (VI) wherein X is Cl and A is -CF 3 The preparation methods include:
[0032] (1) In the presence of a suitable acid catalyst, the compound of formula (I) is reacted with the compound of formula (VII), wherein: R 1 for H, C 1-6 Alkyl or C 1-6 Alkylcarbonyl, R 2 for H, R 3 for H, -CH 3 ,-CH 2 CH 3 or by 0-3 R 12 Substituted phenyl, R 4 , R 5 , R 4a , R 5a with R 6 independently selected from H, C 1-6 Alkyl, C 1-6 Alkoxy and C 1-6 Alkylthio, and R 12 for H, C 1-6 Alkyl, C 1-6 Alkoxy or C 1-6 Alkylthio; generate formula (II) compound;
[0033] (2)(a) reacting 1R, 2S-pyrrolidinyl norephedrine with n-hexyl lithium and cyclopropylacetylene to form a mixture of 1R, 2S-pyrrolidinyl norephedrine and lithium cyclopropylacetylide, and
[0034] (b) reacting the mixture of step (2)(a) with the compound of formula (II) to generate the compound of formula (III);
[0035] (3) reacting the compound of formula (II...
Embodiment 1
[0169] Preparation of N-(4-chlorophenyl)-2,2-dimethylpropanamide
[0170] 4-Chloroaniline (52.7kg, 413mol) was dissolved in a mixture of tert-butyl methyl ether (180kg), 30% aqueous sodium hydroxide solution (61.6kg, 463mol) and water (24.2kg), then cooled to 15°C. To the resulting slurry was added trimethylacetyl chloride (52.2 kg, 448 mol) over 1 hour while maintaining the temperature below 40°C. After stirring at 30°C for 30 minutes, the slurry was cooled to -10°C for 2 hours. The product was collected by filtration, washed with a solution of water (90) dimethanol (10) (175 kg), and dried in vacuo to give 85 kg (97% yield) of the title compound as a crystalline solid, mp. 152-153°C.
[0171] 1 H NMR (300MHz, CCDCl 3 )δ7.48(d, J=9Hz, 2H)7.28(d, J=9Hz, 2H); 13 C NMR (75MHz, CDCl 3 )d 176.7, 136.6, 129.1, 128.9, 121.4, 39.6, 27.6.
[0172] Preparation of N-(4-fluorophenyl)-2,2-methylpropanamide
[0173] A person familiar with the field of organic synthesis understands t...
Embodiment 2
[0175] Preparation of 4-chloro-2-trifluoroacetanilide hydrochloride hydrate
[0176] N-(4-chlorophenyl)-2,2-dimethylpropanamide (36.7kg, 173mol) was added to a solution of TMEDA (20.2kg, 174mol) in anhydrous tert-butyl methyl ether (271.5kg), and Cool to -20°C. To this cooled slurry was added 2.7N n-butyl lithium in hexane (101.9 kg, 393 mol) while maintaining the temperature below 5°C. After cooling at 0-5°C for 2 hours, the solution was cooled to below -15°C and reacted rapidly with ethyl trifluoroacetate (34.5kg, 243mol). After 10 minutes the resulting solution was poured into 3N HCl (196 L, 589 mol) keeping the temperature below 25 °C. After removal of the aqueous phase, the organic solution was concentrated and about 200 L of solvent was distilled off. Acetic acid (352 kg) was added while 325 kg of solvent was distilled off at 100 mm Hg. After the solution was cooled to 30°C, 12N HCl (43.4 kg, 434 mol) was added and the mixture was heated to 65-70°C for 4 hours. The ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com