Novel 3,5 and/or 6 substituted analogues of swainsonine, process for their preparation and their use as therapeutic agents
A halogen, representative technology, applied in the field of swainsonine analogs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
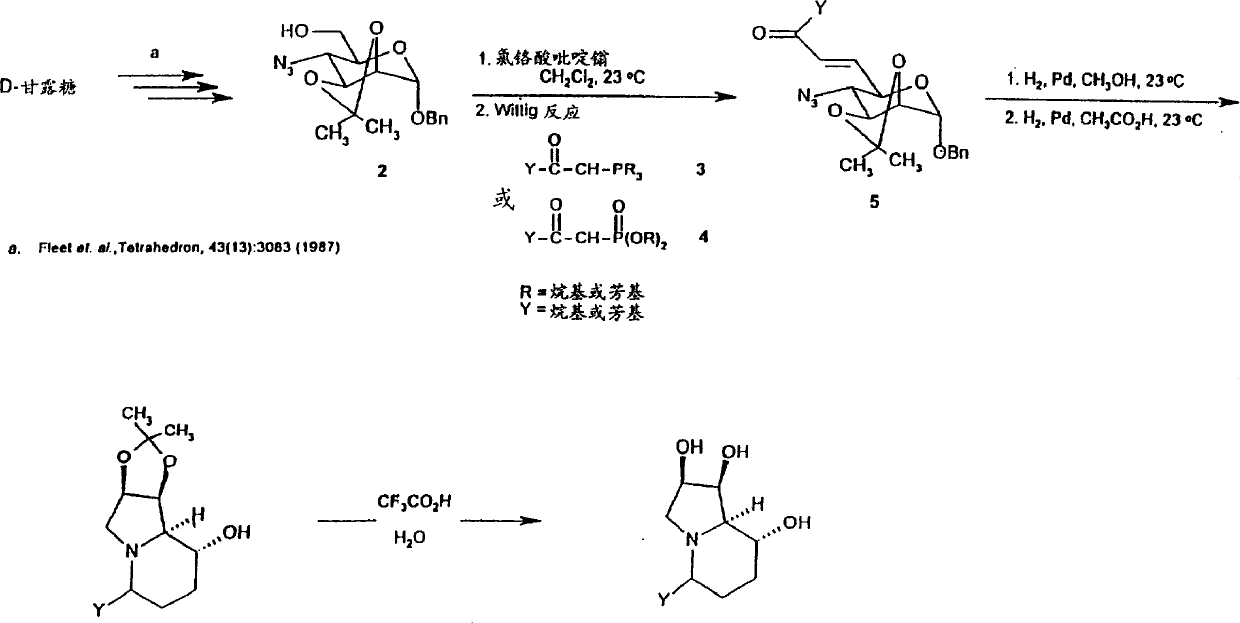
[0130] Pyridinium chlorochromate (776 mg, 3.6 mmol) was added to flame-dried (flame-dried) 3 Å molecular sieves (3 g) and benzyl 4-azido-2,3-O-isopropylidene-α- In a vigorously stirred suspension of D-mannopyranoside (500mg, 1.49mmol) in anhydrous dichloromethane (50ml). After 30 minutes, the oxidation was complete and the slurry was placed on top of a silica gel column (50 g) and eluted with 1:1 ethyl acetate:hexanes. The aldehyde-containing eluate was concentrated, the residue was dissolved in anhydrous benzene (30ml), the solution was cooled to 0°C, and triphenylphosphoranylidene-2-propanone (1.2g, 3.77mmol) was added Anhydrous tetrahydrofuran (30ml) solution. The solution was stirred overnight at room temperature. The reaction mixture was concentrated to dryness and the residue was purified by chromatography (16 g silica gel, 1:3 ethyl acetate:hexanes) to afford GDLZ1 (340 mg, 61%) as a spontaneously crystalline syrup. Benzyl 4-azido-4,6,7,9-tetradeoxy-2,3-O-isopropylid...
Embodiment 2
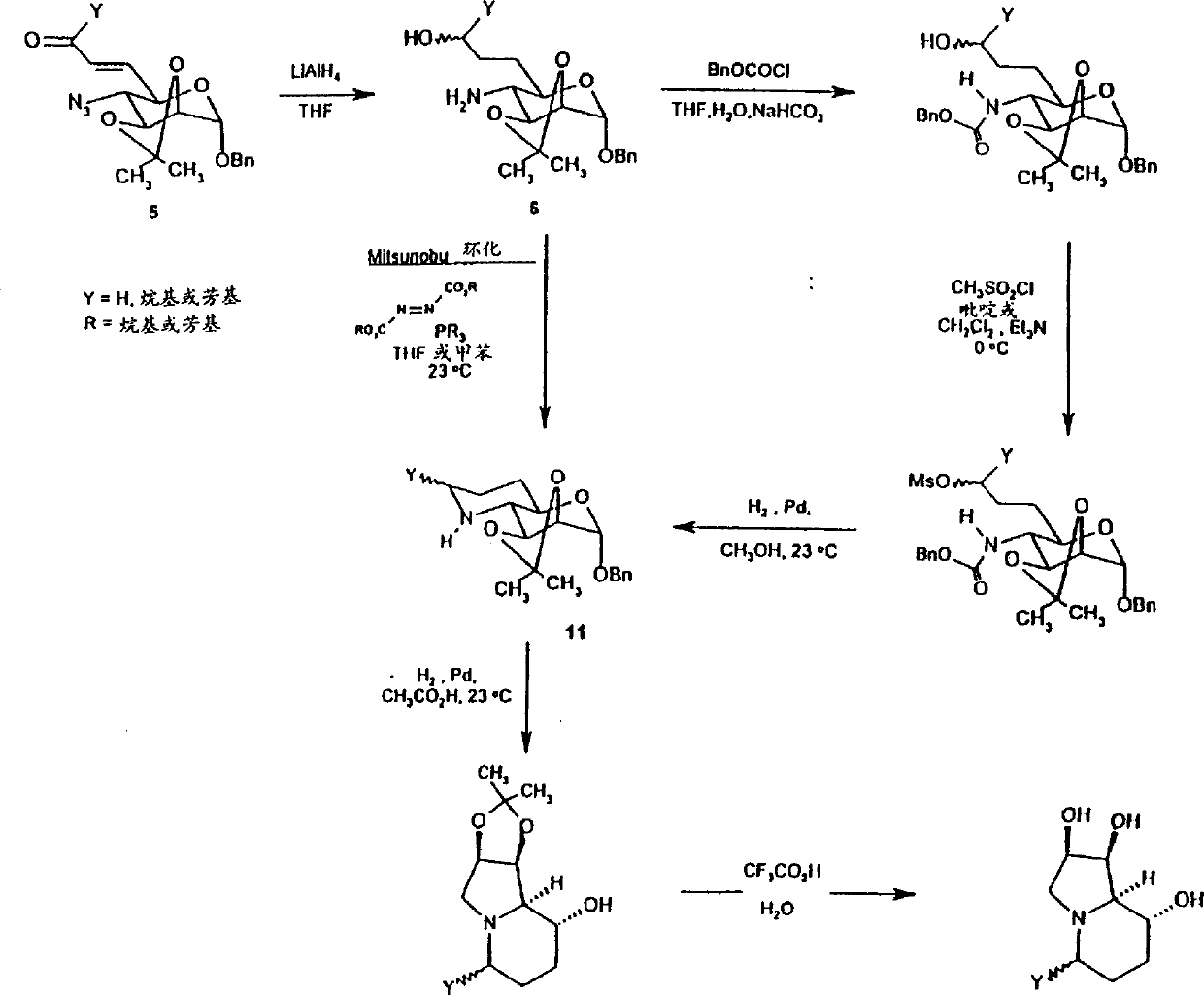
[0163] Stirring into benzyl-4-azido-4-deoxy-2,3-O-isopropylidene-α-D-mannopyranoside (1.0 g, 3.0 mmol) in dry THF (10 ml), Add LiAlH to the cooled solution (in an ice bath at 0°C) in small amounts and several times 4 (140 mg, 3.8 mmol). Then the reaction solution was slowly warmed to room temperature. After 4 hours, TLC indicated complete consumption of starting material and formation of a single new product. with 5% NH 4 The reaction was quenched with Cl (10ml), followed by liquid-liquid extraction (H 2 O / CH 2 Cl 2 )deal with. dry (MgSO 4 ) and the combined organic extracts were concentrated to afford the desired amine 1 (0.90 g, 2.9 mmol) as a white crystalline solid in 97% yield. Benzyl-4-benzyloxycarbonylamino-4-deoxy-2,3-O-isopropylidene-α-D-mannopyranoside (2):
[0164] To 1 (215 mg, 0.70 mmol) (1:1) THF:10% NaHCO 3 (15ml) was added dropwise to a stirred, cooled (in ice bath at 0°C) solution of benzyl chloroformate (0.11ml, 0.75mmol). The reaction was then slo...
Embodiment 3
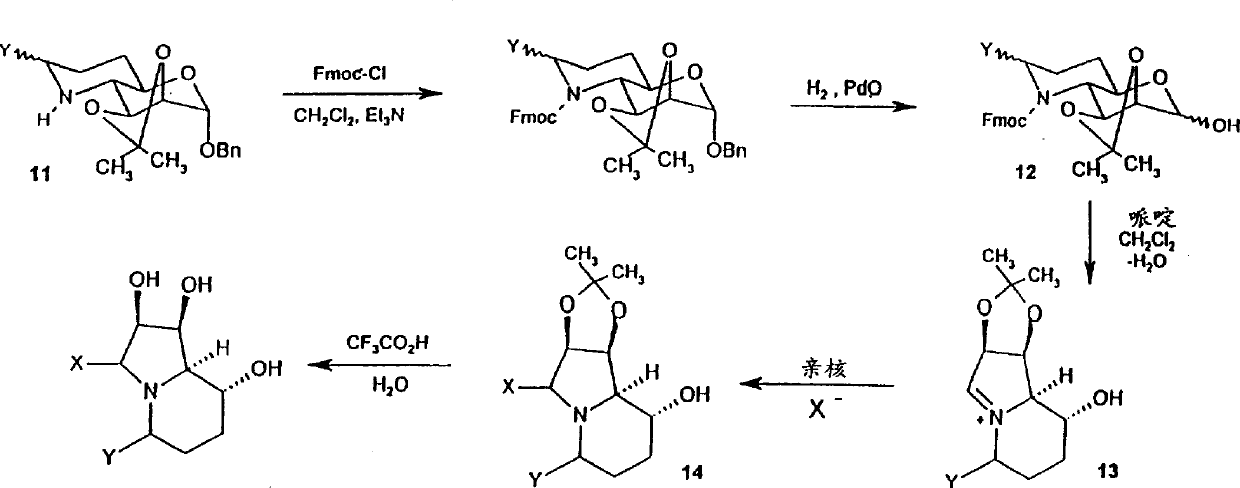
[0172] GD42
[0173] (5R)-5-Benzyloxymethyl swainsonine (5R)-5-Benzyloxymethyl-1,2-O-isopropylidene swainsonine (GDLZ177)
[0174] Sodium hydride (10 mg, 60% in mineral oil) was added in portions to crude (5R)-5-hydroxymethyl-1,2-O-isopropylidene swainsonine (30.7 mg, <0.126 mmol) A solution of benzyl chloride in anhydrous DMF (200ml). After stirring for 2 days, methanol (1 ml) was added and the solution was concentrated. The residue was purified by reverse phase HPLC and GDLZ177 (7.0 mg, about 17%) was obtained. (5R)-5-Benzyloxymethyl swainsonine (GD42)
[0175]Compound GDLZ177 (7.0 mg, 21 mmol) was dissolved in tetrahydrofuran (1 ml), 6M hydrochloric acid (1 ml) was added, and the solution was stirred at room temperature for 2 days. The solution was concentrated to dryness and the residue was purified by reverse phase HPLC to give a colorless residue GD42 (2.3 mg, 38%). (5R)-5-Benzyloxymethyl-1,2-O-isopropylidene swainsonine (GDLZ252)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com