Carboxylic side group containing high performance polyaryl ether copolymer and its preparation
A polyarylether, high-performance technology, applied in the field of polymer materials, can solve the problem of low Tg
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
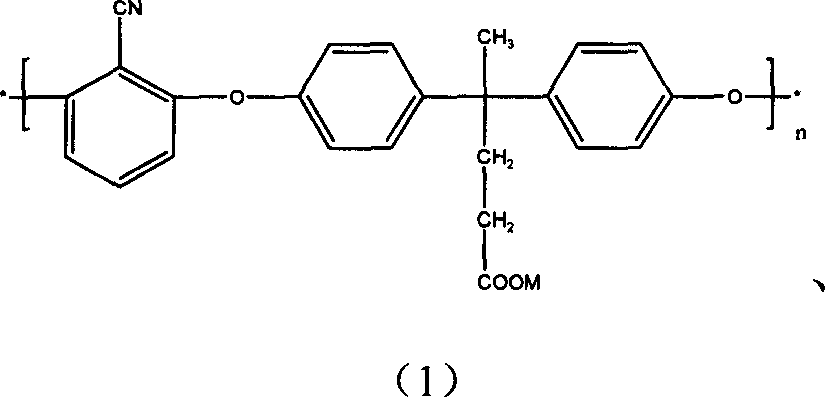
[0038] Example 1: 28.63 grams (0.1mol) of 4,4'-di(p-hydroxyphenyl)-2-pentanoic acid, 13.9 grams (0.1mol) of 2,6-difluorobenzonitrile, 24.84 grams (0.18mol) Anhydrous potassium carbonate, 250ml sulfolane (or dimethylformamide, dimethyl sulfoxide, N-methylpyrrolidone, the impact of different solvents on the reaction results is only that the molecular weight of the resulting polymer is not the same), 50ml toluene (different The azeotropic dehydrating agent has no effect on the reaction result) put it into a 500ml three-necked flask equipped with a water device, ventilate nitrogen, heat up and stir, reflux for 1.5-2 hours, remove toluene, and heat up to 210°C. The reaction was continued at around 210°C for 5 hours.
[0039]If the reacted solution is poured into deionized water, crushed, washed, and dried, polyarylether nitrile containing potassium salt is obtained; The method is acidified in a solvent for 12 hours, then poured into deionized water, pulverized, washed and dried to...
Embodiment 2
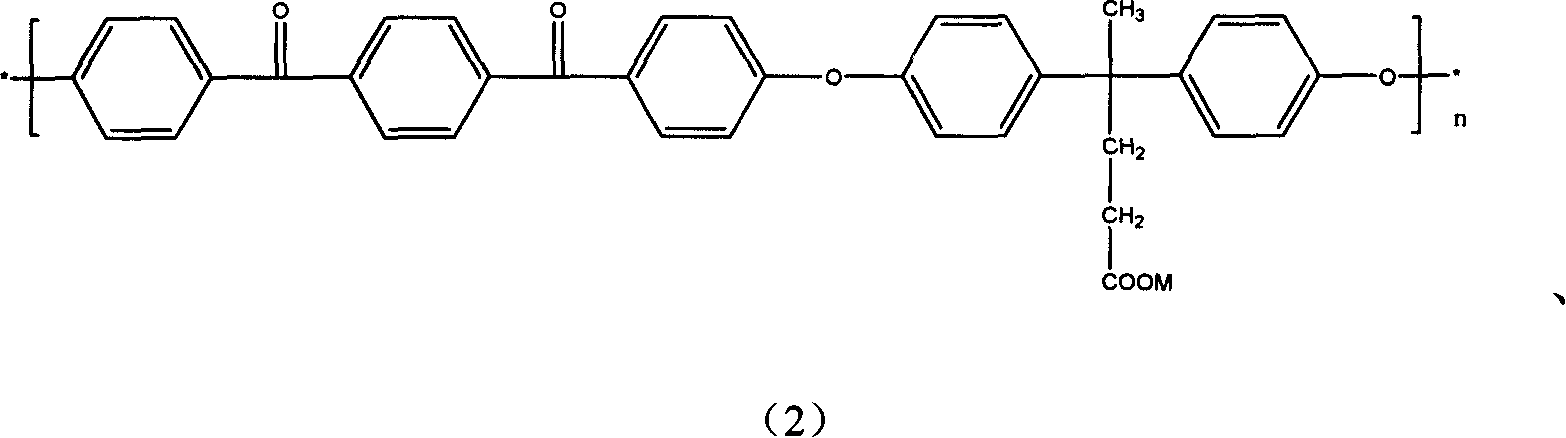
[0041] Example 2: 2,6-difluorobenzonitrile in Example 1 is replaced with 32.2 grams (0.1 mol) of 1,4-di-4-fluorobenzoylbenzene, and other conditions are the same, then a polyether with a carboxyl group is obtained ether ketone ketone. The glass transition temperature of the carboxyl-containing material measured by DSC is 167°C, and the reaction formula is as follows:
[0042]
Embodiment 3
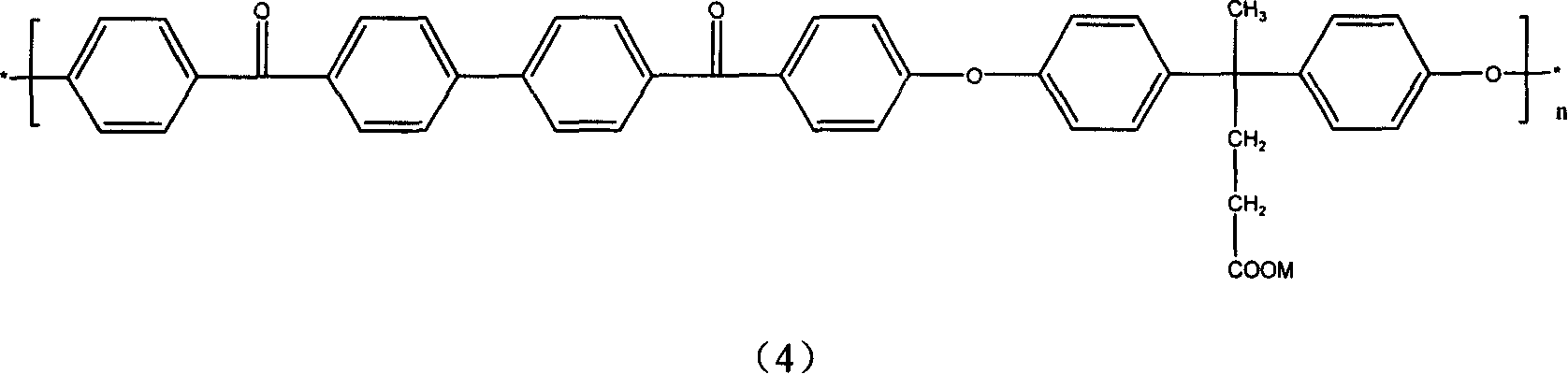
[0043] Example 3: 2,6-difluorobenzonitrile in Example 1 was replaced with 37.2 grams (0.1 mol) of 1,4-di-4-fluorobenzoylnaphthalene, and other conditions were the same to obtain polyether ether with carboxyl groups Keto Keto. The glass transition temperature of the carboxyl-containing material measured by DSC is 176°C, and the reaction formula is as follows:
[0044]
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com