Method for preparing 2-methyl-5, gamma-dioxotetrahydrofuran-2-pentanoic acid by catalysis of levulinic acid on basis of solvent process
A technology of dioxotetrahydrofuran and levulinic acid, applied in directions such as organic chemistry, can solve problems such as poor catalyst stability, low reaction temperature, poor stability, etc., and achieve the effects of reducing adverse effects, wide sources, and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
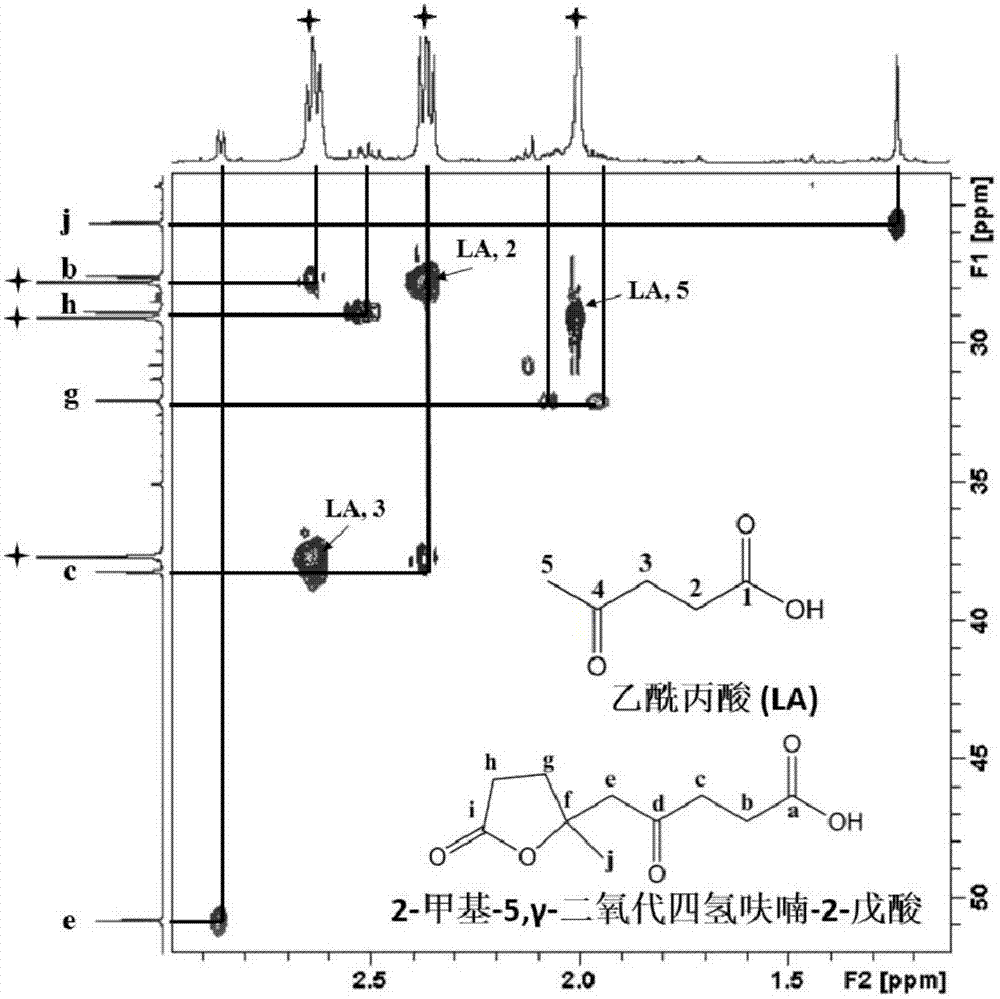
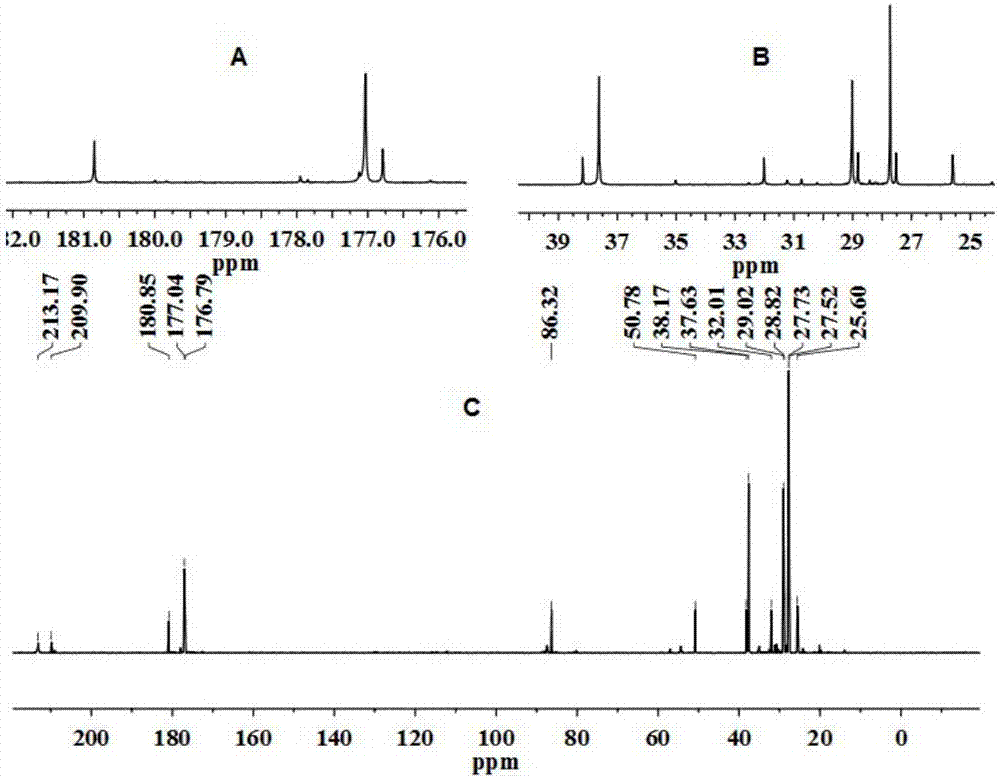
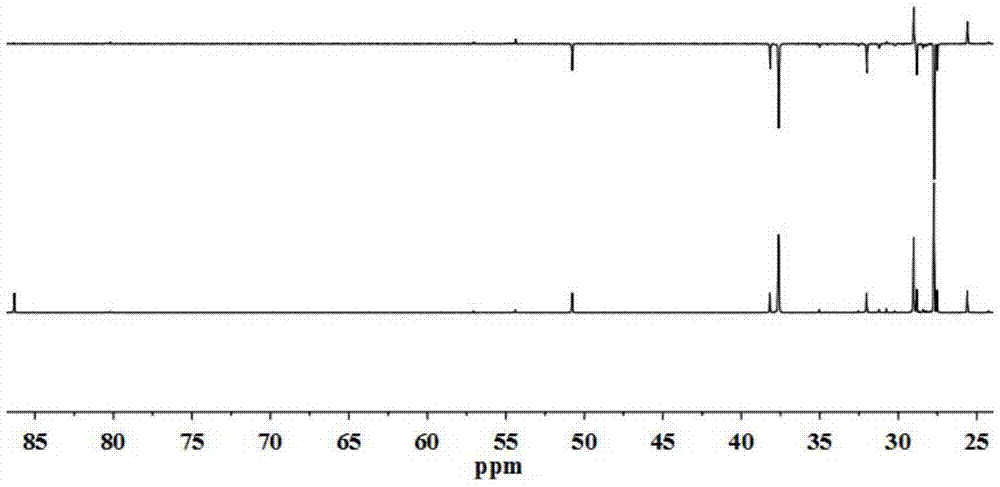
[0027] Mix 10g of levulinic acid and 100ml of γ-valerolactone thoroughly, and then add 1.36g of ZnCl 2 Dubbed the reaction system. The reaction solution was heated to 130°C and reacted for 6h. The structure of 2-methyl-5, γ-dioxotetrahydrofuran-2-pentanoic acid is shown in the attached drawing (see attached for NMR and MS results Figure 1-5 ). The reaction product was distilled under reduced pressure at 150° C. and 3 kPa until no liquid distilled out. The distillation residue was dissolved with 10 ml of acetone, filtered, and the filtrate was evaporated to dryness to obtain 3.8 g of the target product with a purity of 85%. The conversion of levulinic acid was 40%, and the overall yield of the process was 32%.
Embodiment 2
[0029] Mix 10g of levulinic acid and 5ml of γ-butyrolactone thoroughly, and then add 0.2g of ZnBr 2 Dubbed the reaction system. The reaction solution was heated to 120°C and reacted for 6h. The reaction product was distilled under reduced pressure at 140° C. and 2.5 kPa until no liquid distilled out, dissolved in 20 ml of ethyl acetate, filtered, and the filtrate was evaporated to dryness to obtain 4 g of the target product with a purity of 88%. The conversion of levulinic acid was 42%, and the overall yield of the process was 35%.
Embodiment 3
[0031] Mix 10g of levulinic acid and 8ml of γ-valerolactone thoroughly, and then add 0.5g of FeCl 3 Filled into a reaction system. The reaction solution was heated to 70°C and reacted for 96h. The reaction product was distilled under reduced pressure at 130° C. and 2 kPa until no liquid distilled out, dissolved in 30 ml of acetone, filtered, and the filtrate was evaporated to dryness to obtain 4.1 g of the target product with a purity of 85%. The conversion of levulinic acid was 43%, and the overall yield of the process was 35%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com