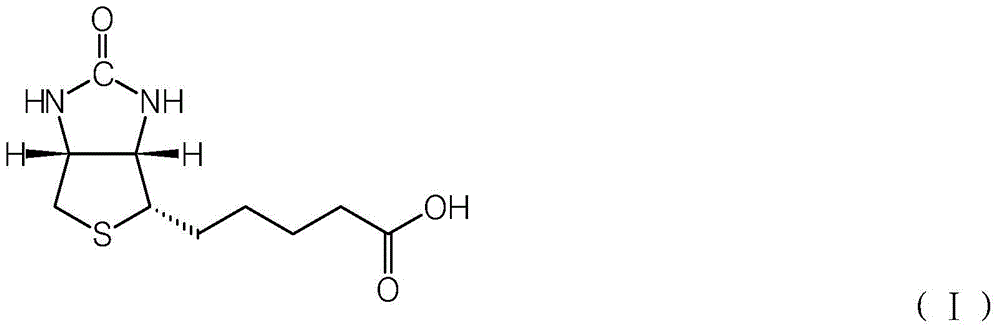
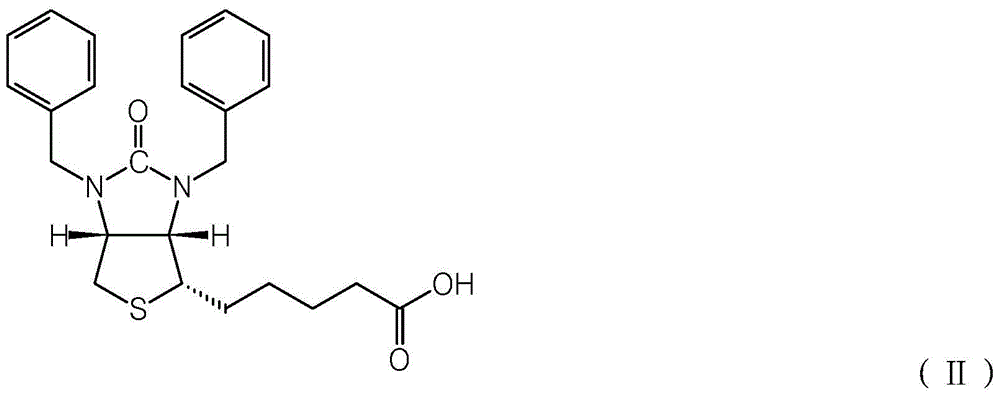
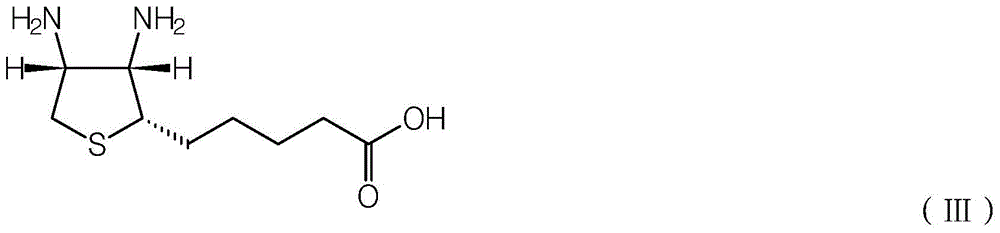
A kind of synthetic method of d-biotin
A synthesis method and biotin technology, applied in the direction of organic chemistry, etc., can solve problems such as potential safety hazards, and achieve the effects of increasing yield, improving reaction efficiency and being environmentally friendly.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] In a 500ml polytetrafluoroethylene-lined autoclave, put in (2S,3S,4S)-5-(3,4-diamino-tetrahydrothiophen-2-yl)valeric acid 30.0g (98.5%, 0.1354mol) , put 200 g of dimethyl carbonate (99.2%, 2.2044 mol), and 3.5 g of boron trifluoride-diethyl ether complex. After feeding, the autoclave is filled with high-purity nitrogen to 0.3MPa, and then exhausted to 0.05MPa, and then replaced twice, for a total of three replacements. After the last replacement, ensure that the residual nitrogen pressure in the autoclave is about 0.05MPa. After the replacement is completed, the electric heating of the autoclave is turned on, and the heating temperature is set to 150°C. When the internal temperature reaches 150°C, the time is started, and the holding reaction is carried out. The pressure at this time is about 0.3-0.6MPa (related to the residual pressure after the last replacement).
[0036]During the reaction, the content of the reaction solution was detected by sampling high-performan...
Embodiment 2
[0048] In a 500ml polytetrafluoroethylene-lined autoclave, put in (2S,3S,4S)-5-(3,4-diamino-tetrahydrothiophen-2-yl)valeric acid 30.0g (98.5%, 0.1354mol) , put into diethyl carbonate 300g (99.0%, 2.5169mol), trisulfomethane (CAS: 54322-33-7) 2.2g. After feeding, the autoclave is filled with high-purity nitrogen to 0.3MPa, and then exhausted to 0.05MPa, and then replaced twice, for a total of three replacements. After the last replacement, ensure that the residual nitrogen pressure in the autoclave is about 0.05MPa. After the replacement, the electric heating of the autoclave was turned on, and the heating temperature was set to 170°C. When the internal temperature reaches 120°C, the time is started, and the heat preservation reaction is carried out. The pressure at this time is about 0.4-0.6MPa (related to the residual pressure after the last replacement).
[0049] During the reaction, the content of the reaction solution was detected by sampling high-performance liquid chrom...
Embodiment 3~14
[0054] In Examples 3 to 14, the reaction was carried out according to the input amounts of the raw materials in Table 1 and the reaction conditions in Table 2, and the other conditions were the same as those in Example 1.
[0055] Table 1 Raw material input amount of Examples 3-14
[0056]
[0057] The reaction conditions and reaction results of Table 2 Examples 3-14
[0058]
[0059] From the results in Table 2, it can be seen that the efficiency of the reaction catalyzed by the Lewis acid is generally higher than that of the protic acid, and the reaction time is shorter.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com