Preparation process of lithium ferrous phosphate for positive pole of lithium ion cell
A lithium iron phosphate and lithium ion battery technology, applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., can solve problems such as low electronic conductivity and ion diffusion rate, inability to form electronic conductivity, and the influence of intercalation and extraction movement , to achieve the effect of improving electrical conductivity, preventing oxidation and reducing particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Mix 0.5 mole of lithium carbonate, 1 mole of ferrous oxalate, 1 mole of ammonium hydrogen phosphate and 10 g of benaphenaline terpolymer, add to the mixing tank, add 200 g of agate balls, seal the mixing tank, and mix on a three-dimensional mixer 5 hours.
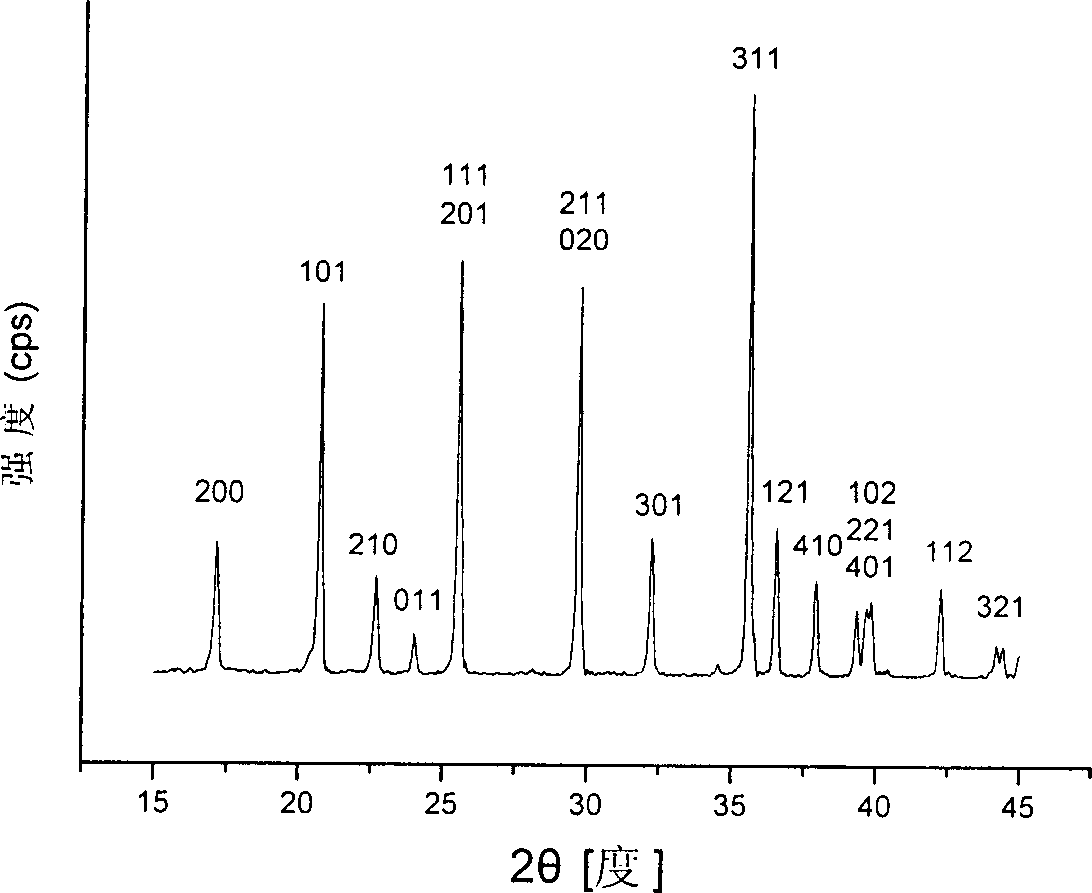
[0027] Put the mixed raw materials in N 2 In the atmosphere protection furnace, under the nitrogen atmosphere of 6L / min, the temperature was raised to 400°C at a rate of 5°C / min, kept at this temperature for 10h, and then raised to 600°C at a rate of 10°C / min, and kept at this temperature Under constant temperature for 30h, then let the furnace cool down to room temperature. figure 1 For the resulting LiFePO 4 The XRD pattern, the addition of additives did not change the LiFePO 4 The crystal structure is maintained with LiFePO 4 The standard XRD spectrum (JCPDS card-190721) has the same crystal structure, all diffraction peaks can be identified according to the standard spectrum, and no impurity peaks of additiv...
Embodiment 2
[0032] Mix 1 mole of lithium acetate, 1 mole of ferrous acetate, 1 mole of ammonium dihydrogen phosphate and 40 g of glucose, add to the mixing tank, add 200 g of agate balls, seal the mixing tank, and mix on a three-dimensional mixer for 10 hours.
[0033] Raise the uniformly mixed raw materials to 300°C at a rate of 10°C / min in an argon atmosphere protection furnace under a nitrogen atmosphere of 9L / min, keep at this temperature for 6h, and then rise to 300°C at a rate of 20°C / min. to 700°C, and kept at this temperature for 20h, then allowed the furnace to cool to room temperature. The electrochemical properties of the obtained positive electrode material were measured according to the method described in Example 1. Figure 5 It is the discharge curve of the material at different current densities. It can be seen from the figure that the synthesized material has excellent high-current discharge performance, and the discharge current is increased by nearly 40 times, and its c...
Embodiment 3
[0035] Mix 1.03 moles of lithium phosphate, 1 mole of ferrous phosphate and 20 g of phenolic resin into a mixing tank, add 200 g of agate balls, seal the mixing tank, and mix on a three-dimensional mixer for 4 hours.
[0036] Raise the uniformly mixed raw materials to 200°C at a rate of 2°C / min in a nitrogen atmosphere protection furnace at 2L / min, keep at this temperature for 30h, and then rise to 200°C at a rate of 5°C / min 600°C, and kept at this temperature for 48h, then allowed the furnace to cool down to room temperature. The electrochemical properties of the obtained positive electrode material were measured according to the method described in Example 1. Figure 6 It is the cycle performance graph of the material. It can be seen from the graph that the synthesized material has excellent cycle performance, and the capacity does not decay after 30 cycles.
[0037] Depend on image 3 It can also be seen that the material has excellent high-temperature charge-discharge an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com