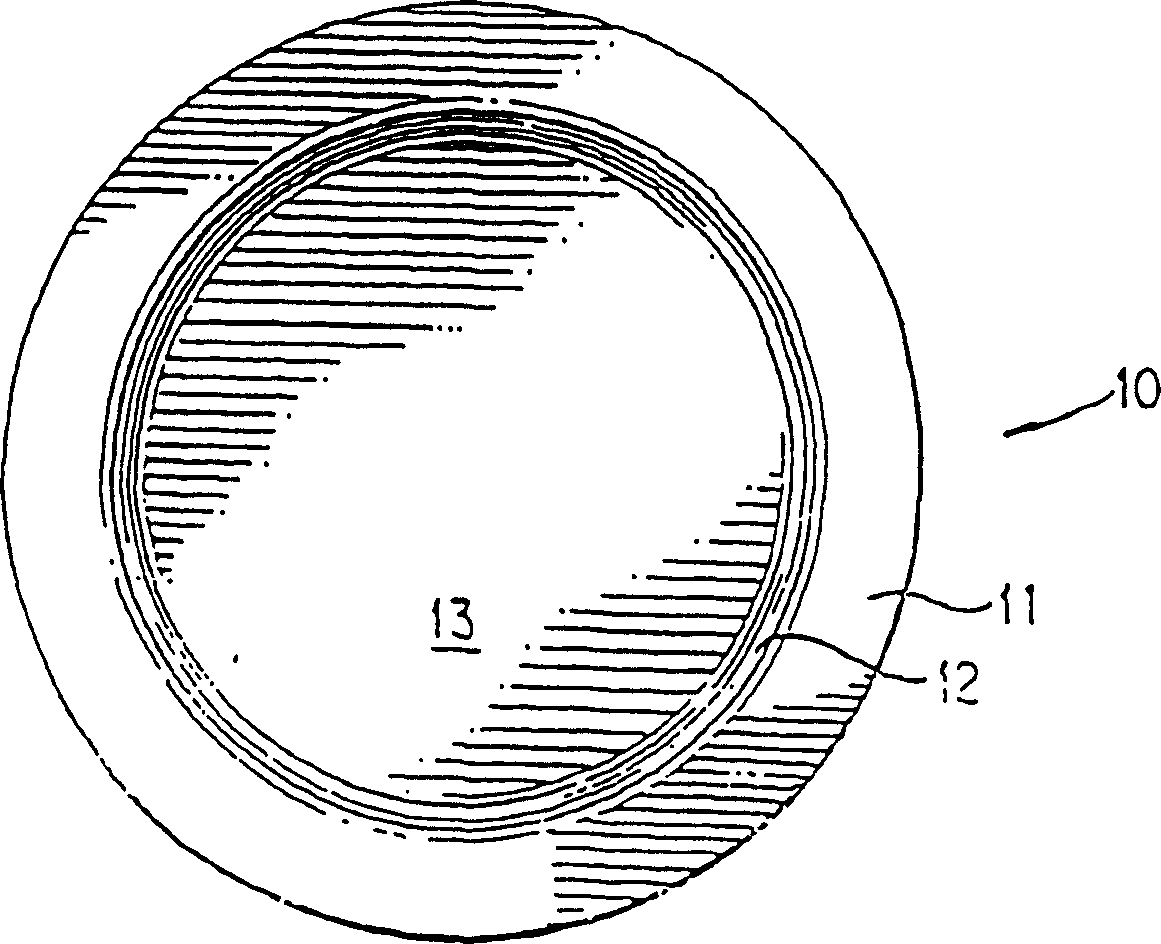
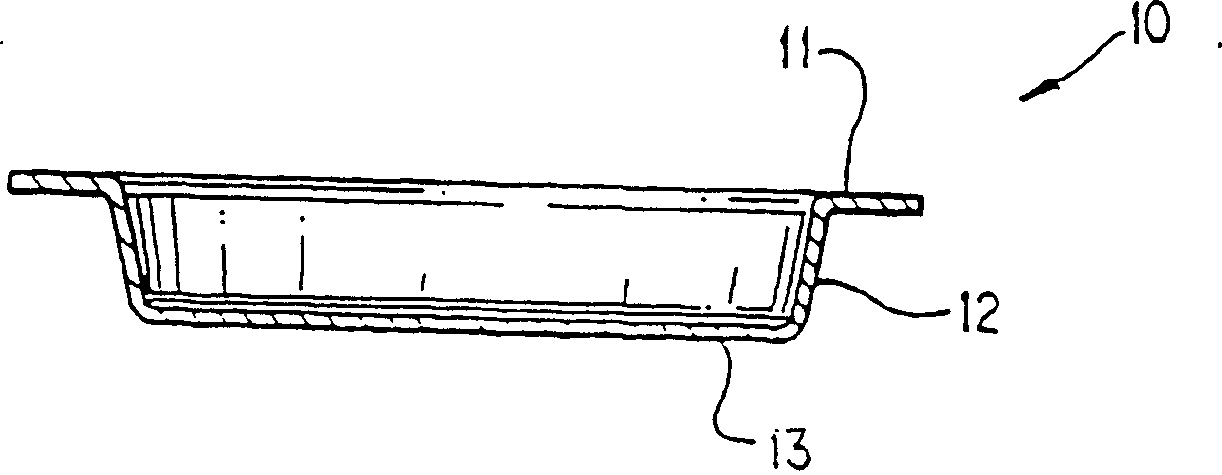
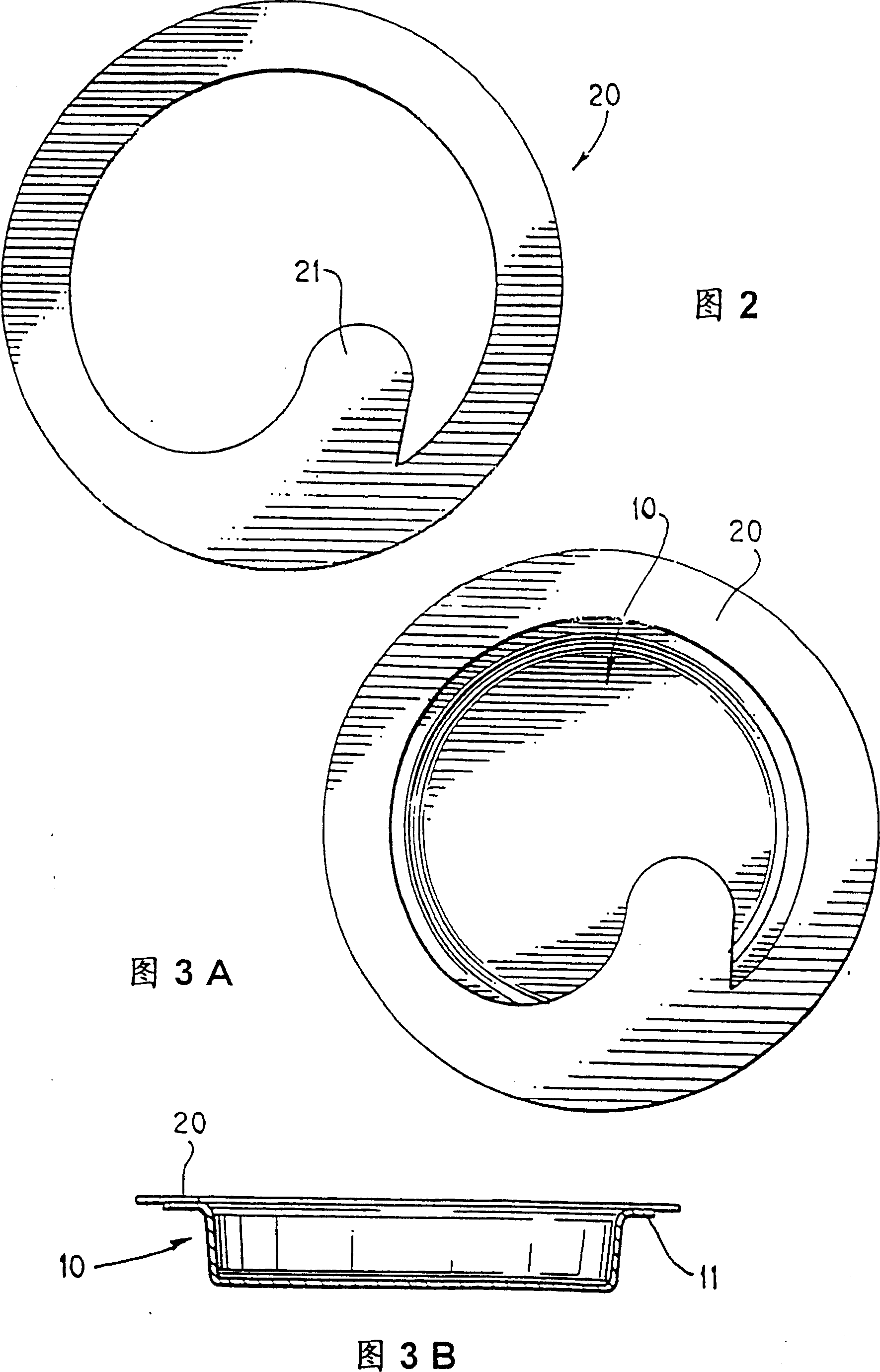
Method and package design for cryopreservation and storage of cultured tissue equivalents
A technology for tissue and refrigeration fluids, used in biological packaging, transportation and packaging, preservation of human or animal bodies, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1 to example 3
[0078] Examples 1 to 3 are refrigeration and thawing techniques with optimal packaging design of the present invention.
example 1
[0079] Example 1: Living skin with packaging design
[0080] Refrigeration of identical (LSE)
[0081] Live skin equivalent (LSE) constructs can be prepared according to US Patent Application Serial No. 08 / 193,809. Nine to 10 days after airlift, LSEs and ligated carrier inserts (TRANSWELL(R), Costar, Cambridge), were placed in 100 mm Petri dishes (Costar). Immerse the construct and transwell in 25 ml of cold storage solution, 2M glycerol in DMEM, in a 100 mm Petri dish for 1 hour to flood the LSE construct with the cold storage solution. During filling, place the construct in a 10% CO 2 Shake at 70 rpm for 1 hour on an orbital shaker (Bellco) in an air chamber. Shaking results in more complete filling and better reproducibility of the refrigeration method. After the LSE was filled with cryogenic fluid, the Petri dish containing LSE, vector insert, and extracellular cryogenic fluid (2M glycerol and DMEM) was placed in a refrigerated box and heat-sealed. In a co-...
example 2
[0085] Example 2: Thawing refrigerated LSE
[0086] The refrigerated LSE was removed from the vapor-phase liquid nitrogen storage, transferred to dry ice and heated to approximately -75°C. After equilibrating at -75°C, transfer the cultured tissue alike to a 37°C water bath; preferably a 4°C water bath; best left at room temperature. Once all of the cold storage solution is seen to be in the liquid phase, the cultured tissue equivalent packaging unit is preferably transferred to a biosafety cabinet, or other sterile location, and washed with ethanol. The lid of the Petri dish is removed by peeling off or the seat of the lid is cut from the bottom of the packaging unit to remove the lid. Excess refrigeration fluid is then poured off and the carrier containing the cultured tissue equivalent is transferred to a Petri dish. Add 25 ml of washing solution (room temperature), preferably DMEM, to the Petri dish containing the cultured tissue equivalent for about 30 minutes to wash aw...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com