Plating metal negative electrodes under protective coatings
A protective layer and electrode technology, applied in battery electrodes, primary battery electrodes, electrode manufacturing, etc., can solve problems such as lack of effective mechanisms for negative electrodes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0108] The following results have been observed:
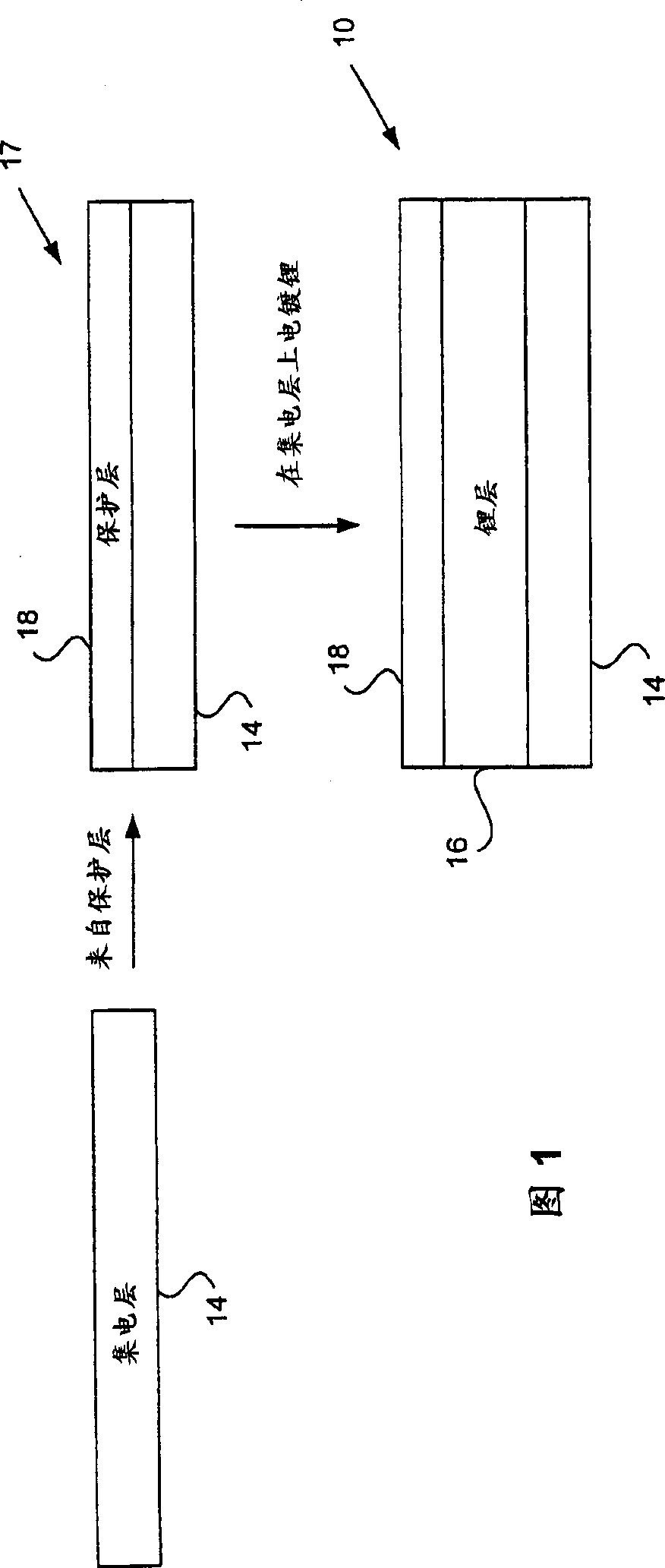
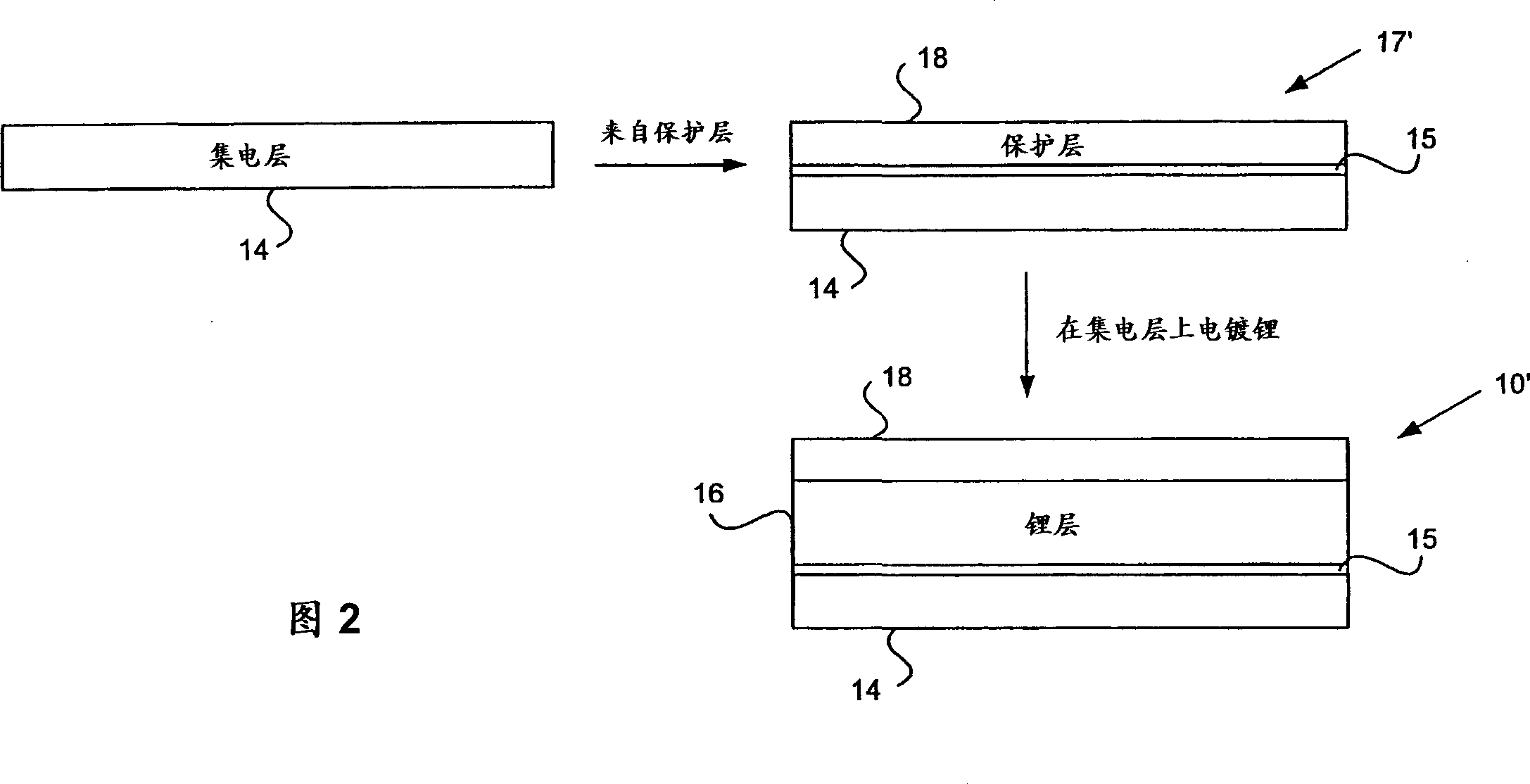
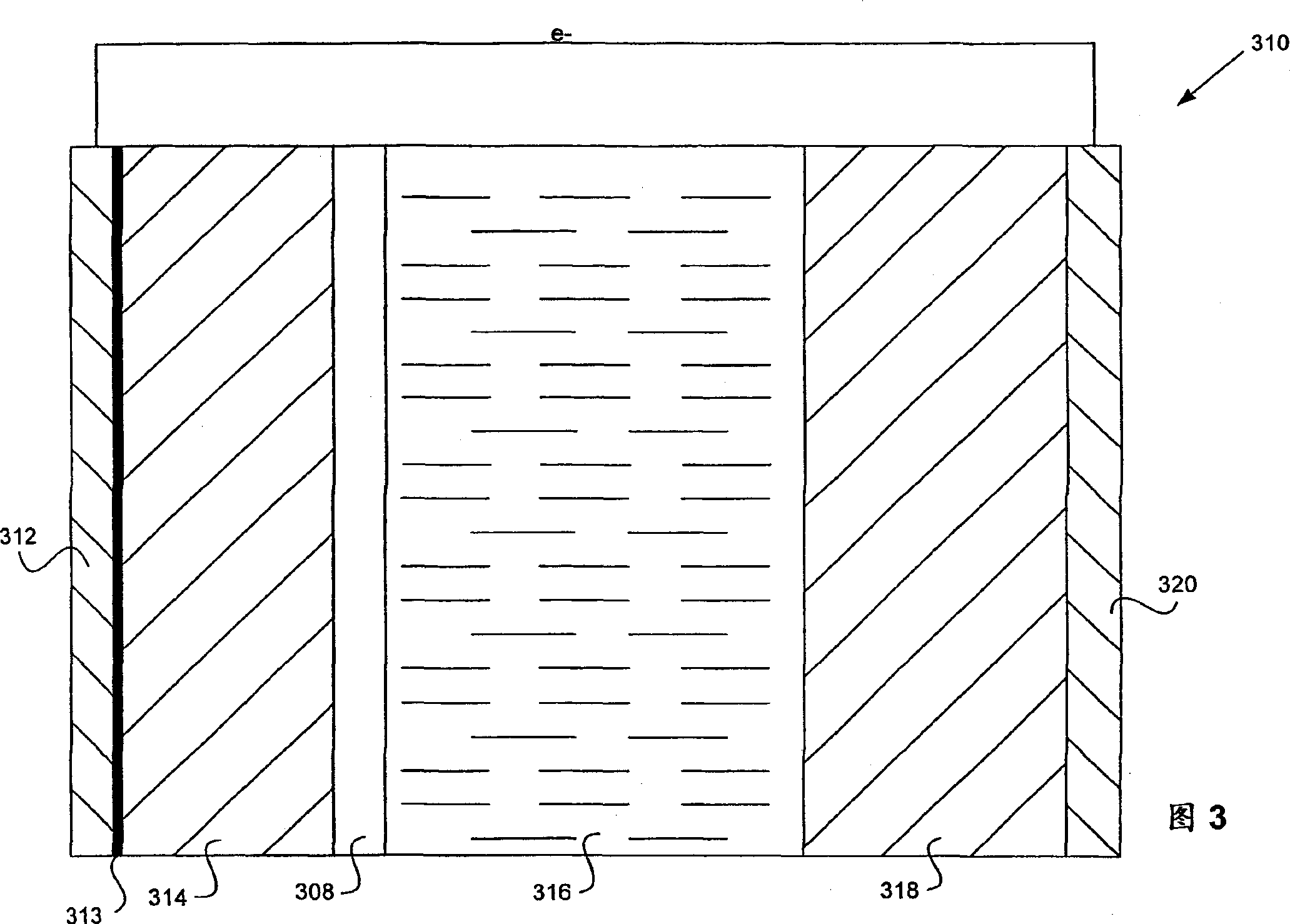
[0109] 1. In an aprotic solvent in the presence of lithium polysulfides, as in the catholyte, luminescent lithium can be electroplated through the LIPON layer onto the copper surface (Example 1). Under similar conditions, electroplating onto bare copper foil would not produce any lithium coating.
[0110] 2. The lithium-plated LIPON / copper structure is available as an anode. Example 2 shows its recyclability in the presence of catholyte.
[0111] 3. The electroplated lithium is still basically fully anode active. Example 3 shows that the lithium availability after 20 cycles is at least 98% of the initial anode active lithium.
[0112] The experimental system was prepared as follows. LIPON / copper composites were made by sputtering LIPON on the copper film, similar to the method described by Bate et al. References are made for this purpose. LIPON targets were prepared by simple melting. By heating 11.5 grams of lithium ph...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com