Disulfide cross-linked glycoprotein hormone analogs, their preparation and use
A technology for glycoprotein hormones and analogs, which is applied in the field of disulfide-bonded glycoprotein hormone analogs and their preparation and application, and can solve problems such as reducing hormone activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0086] Although derivatives are usually prepared by first synthesizing a lead molecule and then derivatizing, derivatives can also be prepared directly. However, the implicit meaning of the term "derivative" is that derivatives are obtained with available techniques by modifying the lead molecule, even if that is not the preferred method of production.
[0087] Modifications to proteins can be divided into the following categories:
[0088] Advantageous - These modifications enhance the utility of the protein for a particular purpose.
[0089] Neutral—These modifications neither enhance nor impair the utility of the protein for a particular purpose.
[0090] Disadvantageous - These modifications impair (but do not necessarily eliminate) the utility of the protein for a particular purpose.
[0091] Inactivation - These modifications eliminate the utility of a specific purpose protein.
[0092] Tolerable - These modifications are favorable, neutral, or unfavorable, but are no...
Embodiment 1
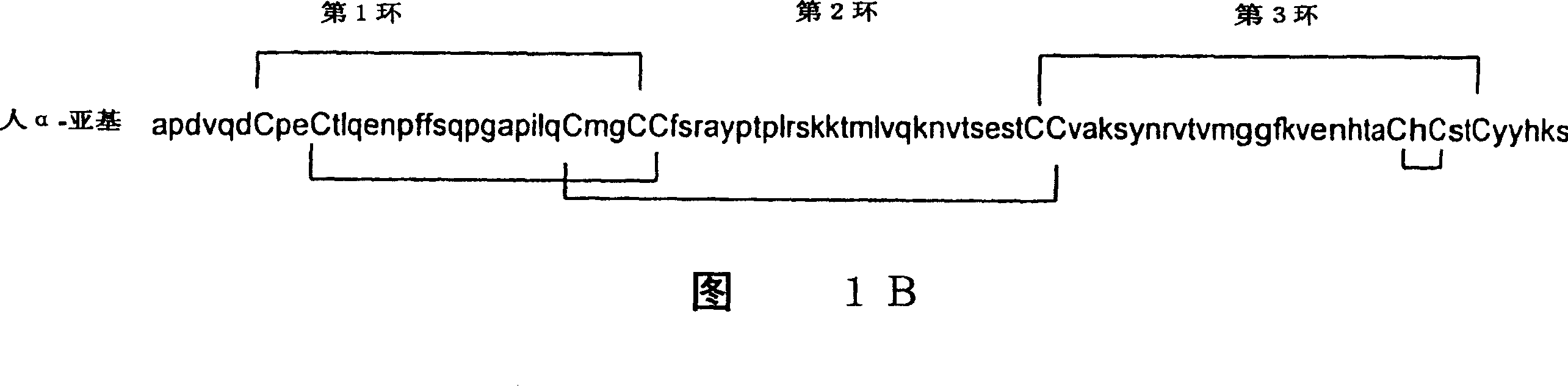
[0254] Example 1: hCG stabilized by intersubunit disulfide bonds between cysteine nodules: hCG(α31-β37)
[0255] Following the criteria for intersubunit disulfide cross-links, the data in Table 1B suggest that if a free cysteine is generated at residue 31 of the α subunit and replaced by a cysteine at Tyr37 in the β subunit, then there is It is possible to make hCG analogs stabilized by intersubunit disulfide bonds between cysteine nodules. This is achieved by making hCG beta subunit analogs in which Tyr37 is replaced by cysteine and human alpha subunit analogs in which Cys7 is replaced by Ser. The latter change disrupts the disulfide bond normally seen between amino acids Cys7 and Cys31 within the α subunit, leaving a free α-subunit cysteinergic at residue 31 and an imported half-cysteine at residue 37 of the β-subunit. Cystine forms disulfide bonds. When each of these subunit constructs is expressed in cultured mammalian cells, the cysteine at residue 31 of t...
Embodiment 2
[0277] Example 2: Multifunctional Glycoprotein Hormone Analogs Stabilized by Intersubunit Disulfide Bonds Located Between the Cysteine Knuckles in the α and β Subunits
[0278] The locus tether is known to affect the receptor binding activity of hCG (Campbell et al., 1991; Moyle et al., 1994; Han et al., 1996). Replacement of hCGβ subunit amino acid residues 101-109 by their hFSH counterparts (i.e., hFSHβ subunit residues 95-103) resulted in a greatly increased FSH activity of the analog without compromising its LH activity (Moyle et al., 1994; Han et al., 1996). In fact, this substitution also enhanced the TSH activity of hCG (Campbell et al., 1997), even though it did not introduce any specific residues seen in hTSH. Studies of the effect of intersubunit disulfide bonds on the activity of related multifunctional analogs show how specific disulfide bonds affect the activity of luteinizing hormone, follicle-stimulating hormone, and thyrotropin. This example describes how i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com