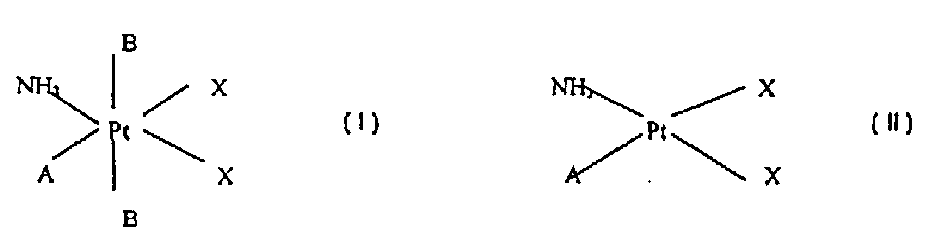Platinum complex, its preparation and therapeutic application
A platinum complex and complex technology, applied in the direction of platinum group organic compounds, platinum group organic compounds, heavy metal compounds, etc., can solve the problem of being unsuitable for oral administration, and achieve broad-spectrum anti-tumor activity and strong efficacy. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Synthesis of af-bis(acetate ion)-b-(l-adamantine)-c-ammine-de-dichloroplatinum(IV) complex (hereinafter referred to as "LA-12")
[0020] Stir 6.25 g (13.3 mmol) of b-(1-adamantine)-c-ammine-de-dichloro-af-dihydroxyplatinum(IV) complex with excess acetic anhydride (50.2 ml, 532 mmol) at room temperature . During this time, solids continued to precipitate from the original solution. After the precipitation had ceased, the solid was filtered and washed with a small amount of acetic anhydride and ether. After drying in a vacuum desiccator, 4.28 g (58.2%) of the title product were obtained.
[0021] The identity of the obtained product was confirmed by 1H and 13C NMR spectroscopy and far infrared spectroscopy, and its purity was confirmed by high performance liquid chromatography.
[0022] Product C 14 h 26 Cl 2 N 2 o 4 Elemental analysis of Pt:
[0023] C(%) H(%) N(%) Cl(%)
[0024] Measured value 30.24 4.75 4.99 12.81
[0025] Calculated value 30.44 4....
Embodiment 2
[0027] af-bis(acetate ion)-b-(l-amino-3,5-dimethyladamantane)-c-ammine-de-dichloroplatinum (IV) complex (hereinafter referred to as "LA-15" )Synthesis
[0028] Stir 0.96 g (1.93 mmol) of b-(1-amino-3,5-dimethyladamantane)-c-ammine-de-dichloro-af with excess acetic anhydride (8 ml, 84.7 mmol) at room temperature - Dihydroxyplatinum(IV) complexes. After dissolution, 10 ml of ether was added to the reaction mixture. Stirring was continued until precipitation ceased. The precipitated solid was filtered, rinsed with ether and dried in a vacuum desiccator to yield 0.72 g (64.3%) of the title product.
[0029] The identity of the obtained product was confirmed by 1H and 13C NMR spectroscopy and far infrared spectroscopy, and its purity was confirmed by high performance liquid chromatography.
[0030] Product C 16 h 30 Cl 2 N 2 o 4 Elemental analysis of Pt:
[0031] C(%) H(%) N(%) Cl(%)
[0032] Measured value 32.88 5.21 4.75 12.31
[0033] Calculated 33.11 5.21 ...
Embodiment 2a
[0035] Synthesis of b-(1-adamantine)-c-ammonia-de-dichloro-af-dihydroxyplatinum (Ⅳ) complex (hereinafter referred to as LA-11)
[0036] 8.01 g (18.44 mmol) of cis-(1-adamantine)-ammonia-dichloroplatinum(II) complex were suspended in 120 ml of water at room temperature. A stoichiometric excess (20 ml) of 30% (w / w) hydrogen peroxide in water was added to the suspension and the reaction mixture was heated at 80°C for 1 hour and then cooled to room temperature. The solid was isolated by filtration, rinsed with water and partially dried. The product was extracted and rinsed with a total of 150 ml of dimethylformamide. Rinse with ether to remove remaining dimethylformamide. After drying in a vacuum desiccator, the yield was 6.45 g, ie 74.6% of theory (relative to the starting platinum(II) complex).
[0037] The identity of the product was determined by far-infrared spectroscopy and its purity was determined by high-performance liquid chromatography.
[0038] The resulting produc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com