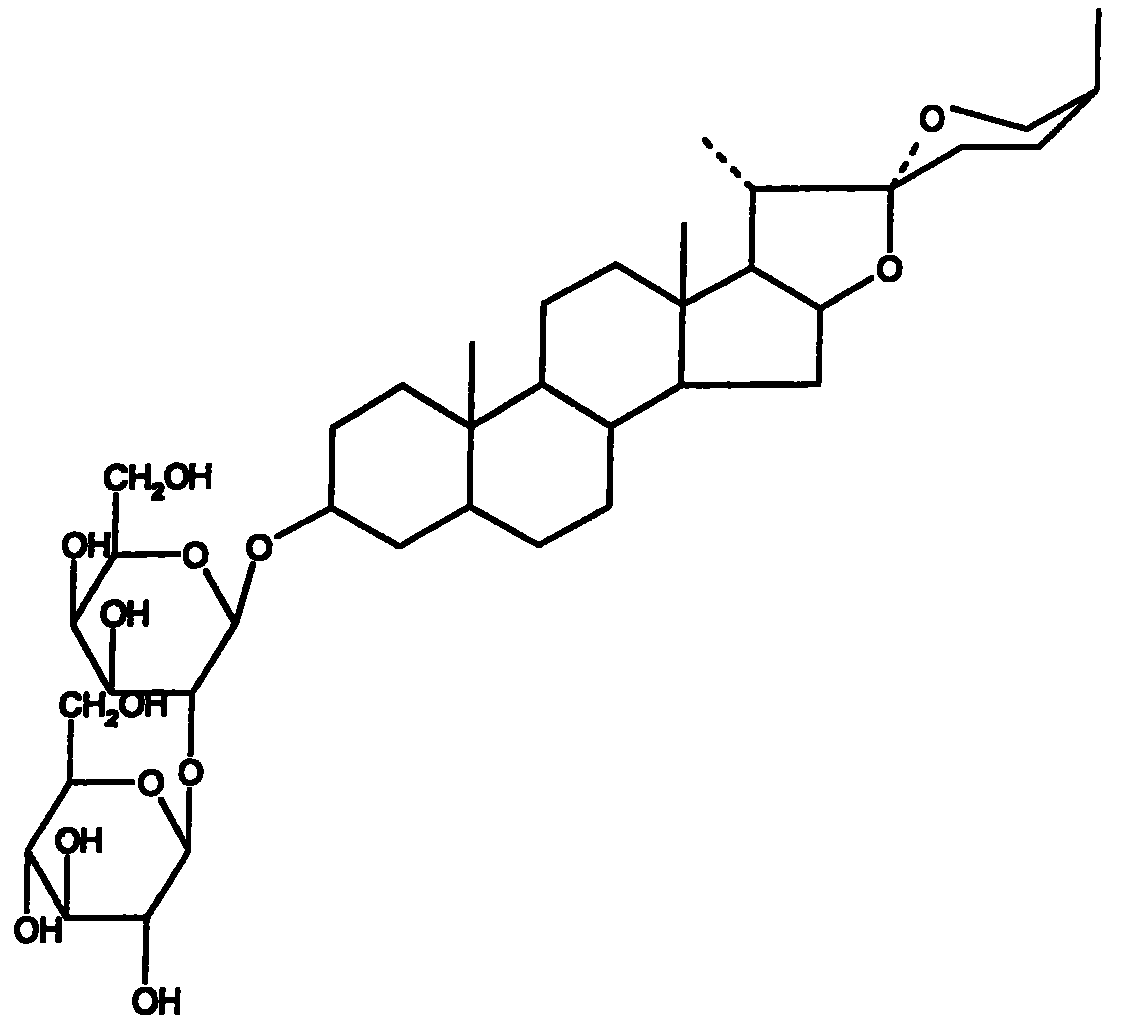
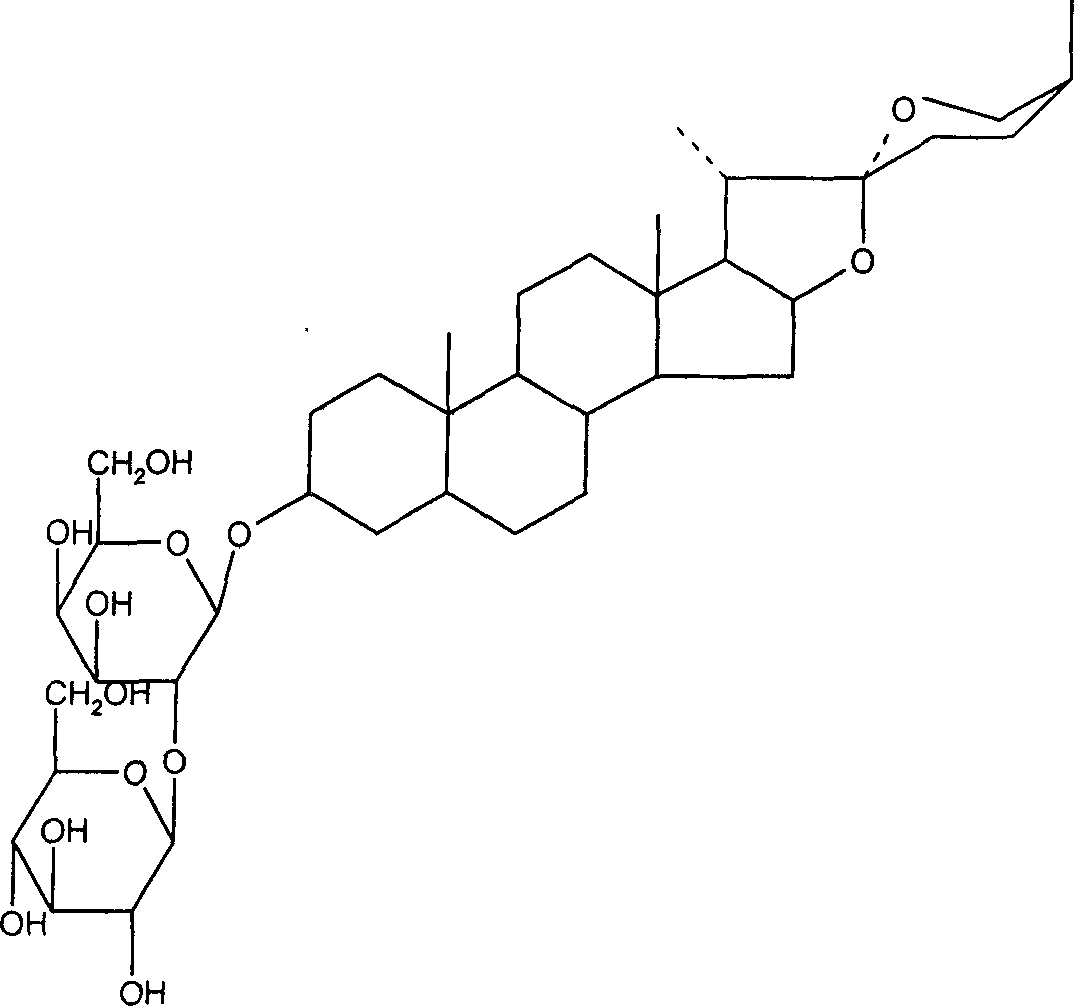
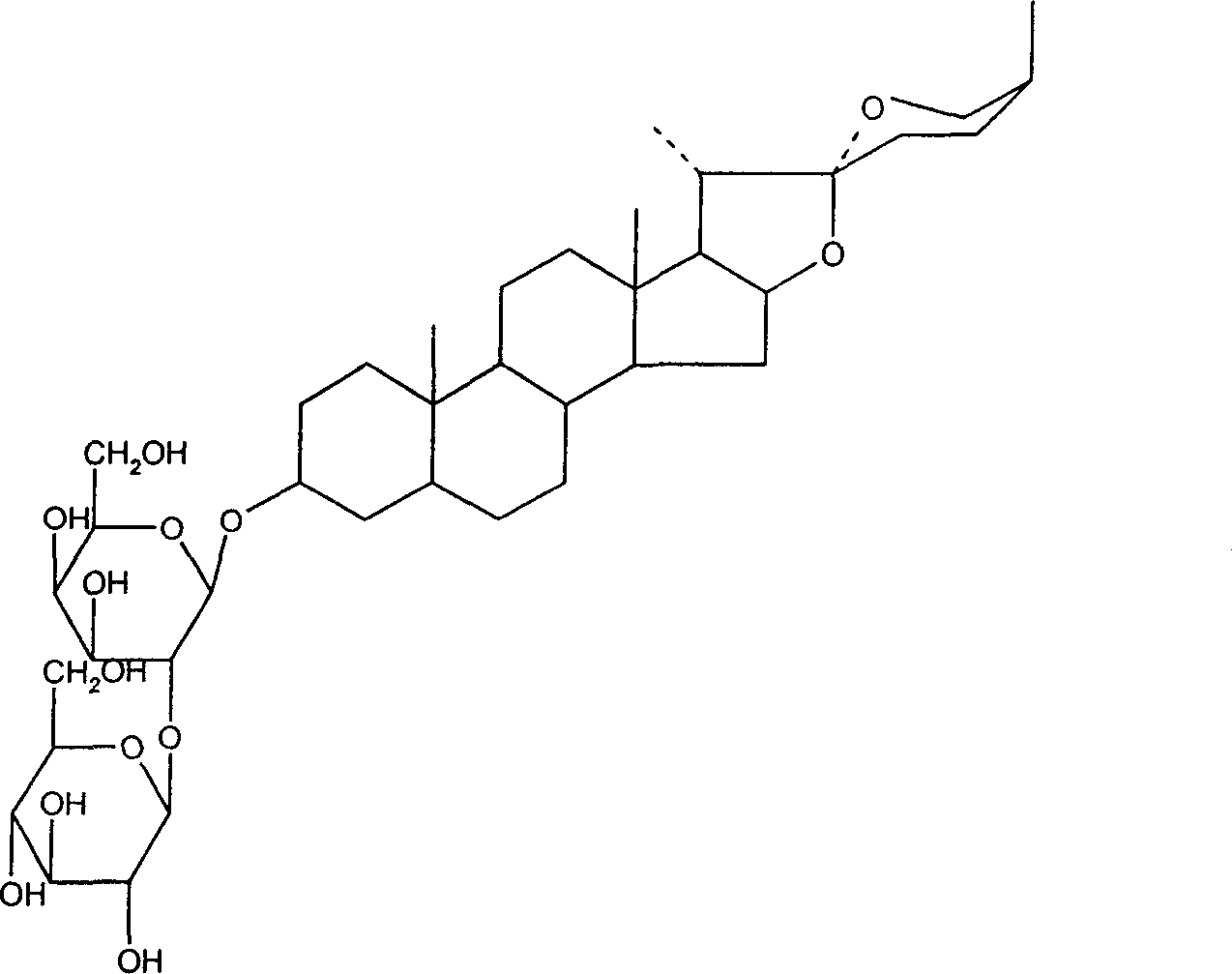
Application of saponins in the preparing process of coxsackie virus resisting medicine
A drug and virus technology, applied in antiviral agents, medical preparations containing active ingredients, drug combinations, etc., can solve the problems of unreported pharmacological effects of monomer components
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: Drug of the present invention is to the impact test (in vivo) of viral myocarditis mouse mortality
[0031] 34 Balb / c mice were randomly divided into 3 groups.
[0032] Normal control group: 6 rats, neither the medicine of the present invention nor virus inoculation, but only an equal amount of distilled water.
[0033] Virus control group: 14 rats, each intraperitoneally inoculated with 0.1ml CVB 3 Virus fluid (TCID 50 =10 -7.5 , 10 5 double dilution).
[0034] Administration group: 14 rats, inoculated with CVB in the same way as above 3 Virus liquid, on the same day, was given the drug of the present invention by intragastric administration at 0.15mmol / kg·d for 7 consecutive days and observed until the 15th day.
[0035] Observe and record the animal's mental state, diet, coat color and death during the dosing (feeding) period.
[0036] Result: the medicine of the present invention can significantly reduce the 3 The mortality rate of mice with vi...
Embodiment 2
[0039] Embodiment 2: the influence test (in vivo) of medicine of the present invention on viral myocarditis mouse cardiac index
[0040] The grouping and dosage of Balb / c mice were the same as in Example 1. After 7 consecutive days of administration, all mice were dissected on the 15th day of observation. The mice were weighed before dissection, and their hearts were weighed after dissection.
[0041] Cardiac index = heart weight / body weight
[0042] When an animal is infected with a virus, its food intake decreases and its body weight decreases, but the heart becomes enlarged, fibrosis and heavier. Therefore, the cardiac index can be used to measure the degree of heart disease. The greater the cardiac index, the more serious the heart disease.
[0043] Result: the medicine of the present invention can significantly reduce the 3 See Table 2 for the heart index of mice with viral myocarditis caused by viruses.
[0044] group
[0045] Note: compared with the admi...
Embodiment 3
[0046] Embodiment 3: the influence test (in vivo) of medicine of the present invention to viral myocarditis mouse pathological section
[0047] The grouping and dosage of Balb / c mice were the same as in Example 1. After 7 days of administration, until the 15th day of observation, the mouse heart was fixed in formalin after dissection, and pathological sections were made. Myocardial inflammatory cell infiltration and myocardial fiber necrosis were observed under a microscope. Observe 5 visual fields for each specimen, record 0-25% lesion in each visual field as 1, 25%-50% as 2, 50%-75% as 3, 75%-100% as 4, if there is no lesion It is recorded as 0.
[0048] Experimental result shows, medicine of the present invention can effectively improve the effect caused by Coxsackie B. 3 The degree of lesion in mouse heart caused by virus.
[0049] group
[0050] Note: Using non-parametric statistical test, comparing the drug treatment group with the virus group, *P<0.05
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com