Group-B type-II Coxsackie virus gene vaccine
A technology of coxsackie virus and gene vaccine, applied in gene therapy, antiviral agents, antibody medical ingredients, etc., can solve the problems of poor safety and poor immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0006] Specific embodiment one: The Coxsackievirus group B type 2 gene vaccine of this embodiment is a pcDNA3-CVB2VP1 eukaryotic expression plasmid composed of the eukaryotic expression system pcDNA3 and CVB2VP1 genes. For the gene sequences of CVB2VP1 and pcDNA3-CVB2VP1, see List of gene sequences.
specific Embodiment approach 2
[0007] Specific implementation mode two: This implementation mode introduces the CVB2 gene vaccine in detail.
[0008] 1. Materials:
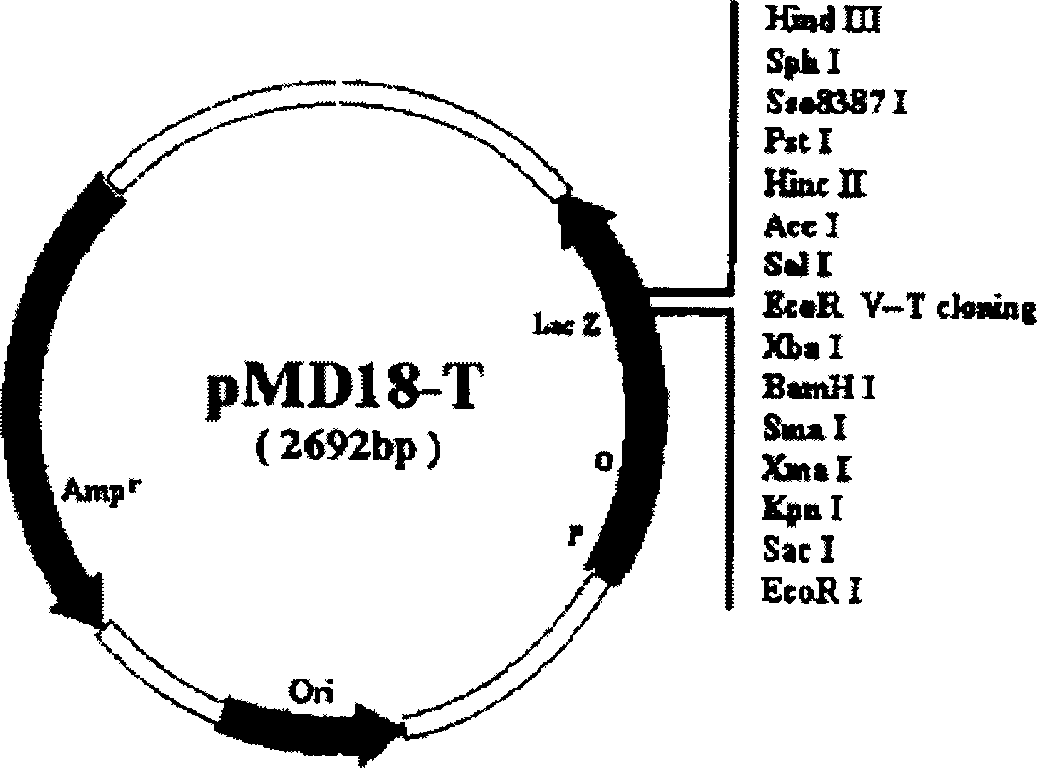
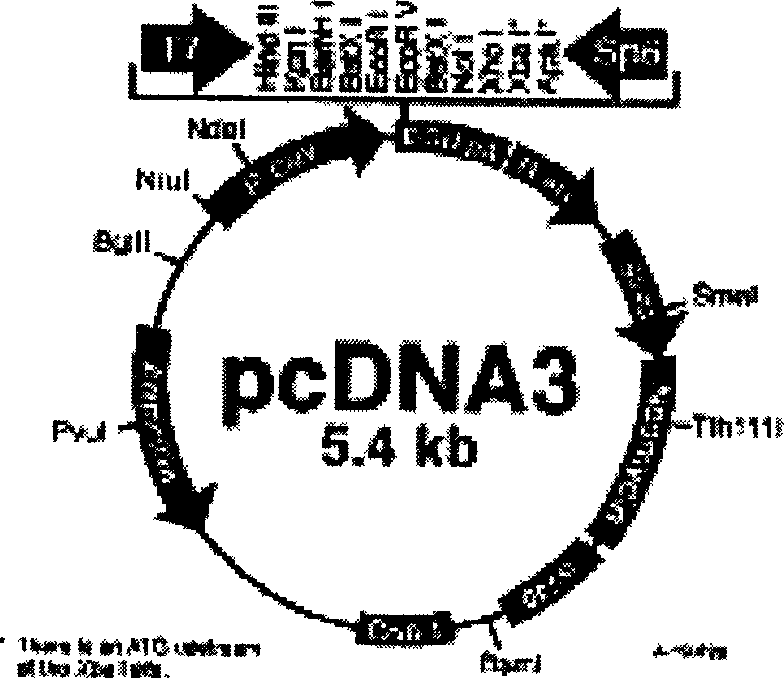
[0009] (1) Viruses, cell lines, vectors and strains: CVB2 Yunnan isolate and HeLa cells were provided by Harbin Medical University. The cloning vector pMD18-T was purchased from TaKaRa Company (such as figure 1 shown), the eukaryotic expression vector pcDNA3 was purchased from Invitrogen (such as by figure 2 shown), the host strain is E.coliJM83, P-815 mouse mastocytoma cells (H-2 d Genotype) was provided by the State Key Laboratory of Biotechnology, Chinese Academy of Agricultural Sciences. The reference strains and GenBank accession numbers are shown in Table 1.
[0010] reference strain
GenBank accession number
Ohio-1
jo / Roma98
fr / Roma98
sp / Roma88
816 / 84
China-1
China-2
MD84-5914
HON84-6016
NC95-2135
FL92-1512
AF081312
AJ309267
AJ309266
AJ309252
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com