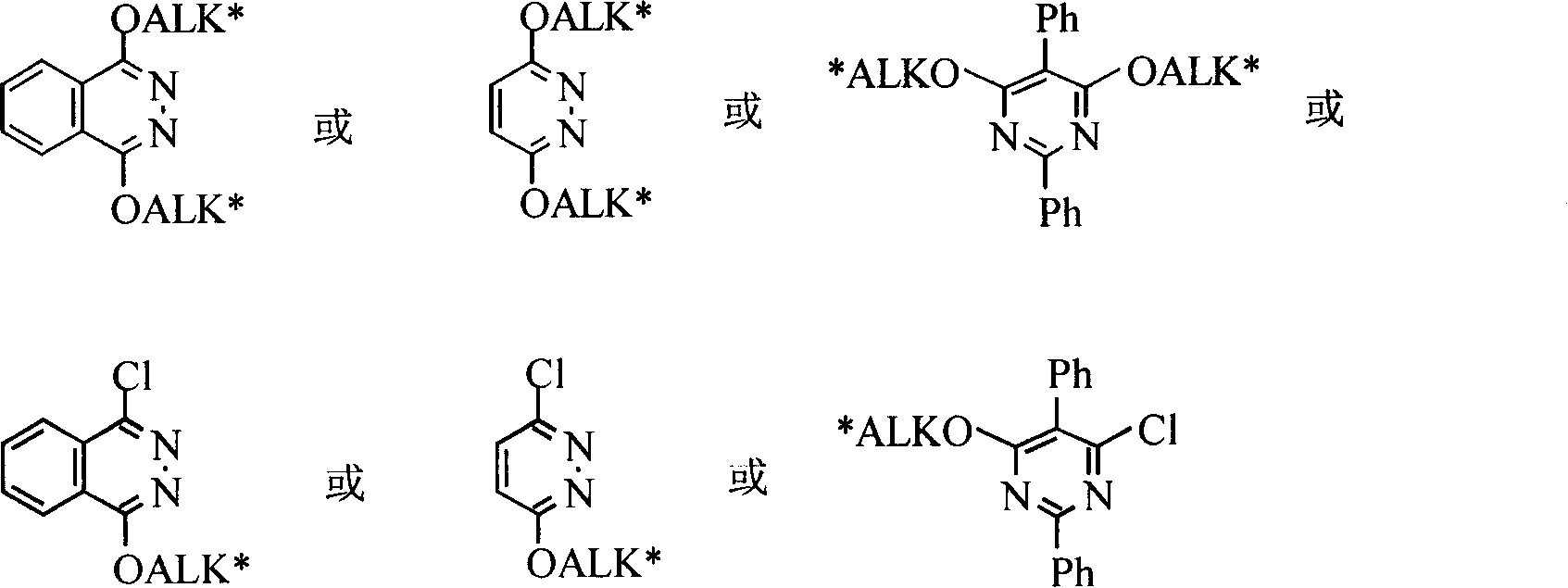
Acid absorbing agent for preparing cinchona alkaloids ligand
A technology for cinchona alkaloids and ligands, which is applied in the field of acid binding agents, can solve the problems of difficulty in accurate quantification, danger, easy combustion, etc., and achieves the effects of avoiding the column chromatography process, easy operation and safe use.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Synthesis of ligand 1
[0027] Add 0.295g (0.0015mol) 1,4-dichloro-2,3-naphthalene, 0.975g (0.003mol) dihydroquinine, 0.63g (0.015mol) CaH into a 100mL three-necked flask 2 , 4.5mL DMF, N 2 Protected and reacted at 90°C. The reaction solution was a pale yellow turbid solution. TLC monitored the reaction process until the raw materials disappeared. The total reaction time was 1 h. Add 10mL ethyl acetate and 5mL water and stir, separate the water layer, extract with ethyl acetate (15mL×3), combine the organic phases, anhydrous MgSO 4 dry. When about 3 mL of liquid was left after filtration and concentration under reduced pressure, white needle-like crystals were deposited, drained, and washed with a little anhydrous ether to obtain white crystals. The yield was 95%. m.p.133~135℃. 1 H NMR(CDCl 3 , Internal standard TMS): δ8.65(d, J=4.6Hz, 2H), 8.33(m, 2H), 7.98(d, J=9.2Hz, 2H), 7.96(m, 2H), 7.56(d, 2H), 7.44(d, J=4.7Hz, 2H), 7.36(d, 1H), 7.35(d, 1H), 7.01(d, 2H), 3....
Embodiment 2
[0028] Example 2: Synthesis of ligand 2
[0029] Add 0.295g (0.0015mol) 1,4-dichloro-2,3-naphthalene, 0.975g (0.003mol) dihydroquinidine, and 0.63g (0.015mol) CaH in a 100mL three-necked flask 2 , 4.5mL N, N-dimethylformamide (DMF), N 2 Protected and reacted at 90°C. The reaction liquid was a pale yellow turbid solution. The reaction process was monitored by TLC until the raw materials disappeared. The total reaction time was 5 hours. Add 10mL ethyl acetate and 5mL water and stir, separate the water layer, extract with ethyl acetate (15mL×3), combine the organic phases, anhydrous MgSO 4 dry. When about 3 mL of liquid was left after filtration and concentration under reduced pressure, white needle-like crystals were deposited, drained, and washed with a little anhydrous ether to obtain white crystals. The yield was 78%. m.p.176~178℃. 1 H NMR(CDCl 3 , Internal standard TMS): δ8.65(d, J=5.3Hz, 2H), 8.33(m, 2H), 7.98(d, J=9.2Hz, 2H), 7.94(m, 2H), 7.58(d, 2H), 7.43(d, J=5.2Hz, 2H), 7.3...
Embodiment 3
[0030] Example 3: Synthesis of Ligand 3
[0031] Add 0.295g (0.0015mol) 1,4-dichloro-2,3-phthalazinone, 0.97g (0.003mol) quinine, 0.63g (0.015mol) CaH into a 100mL three-necked flask 2 , 4.5mL DMF, N 2 Protected and reacted at 90°C. The reaction liquid was a pale yellow turbid solution. The reaction process was monitored by TLC until the raw materials disappeared. The total reaction time was 6 hours. Add 10mL ethyl acetate and 5mL water and stir, separate the water layer, extract with ethyl acetate (15mL×3), combine the organic phases, anhydrous MgSO 4 dry. When about 3 mL of liquid was left after filtration and concentration under reduced pressure, white needle-like crystals were deposited, drained, and washed with a little anhydrous ether to obtain white crystals. The yield was 95%. m.p.160~161℃. 1 H NMR(CDCl 3, Internal standard TMS): δ8.65(d, J=5.3Hz, 2H, Ar-H), 8.33(m, 2H, Ar-H), 7.98(d, J=9.2Hz, 2H, Ar-H) , 7.96 (m, 2H, Ar-H), 7.58 (s, 2H, Ar-H), 7.44 (d, J=5.2 Hz, 2H, Ar-H)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com