Nanoparticles with polymer shells
A technology of nanoparticles and polymers, applied in the field of nanoparticles, can solve problems that are poorly understood
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
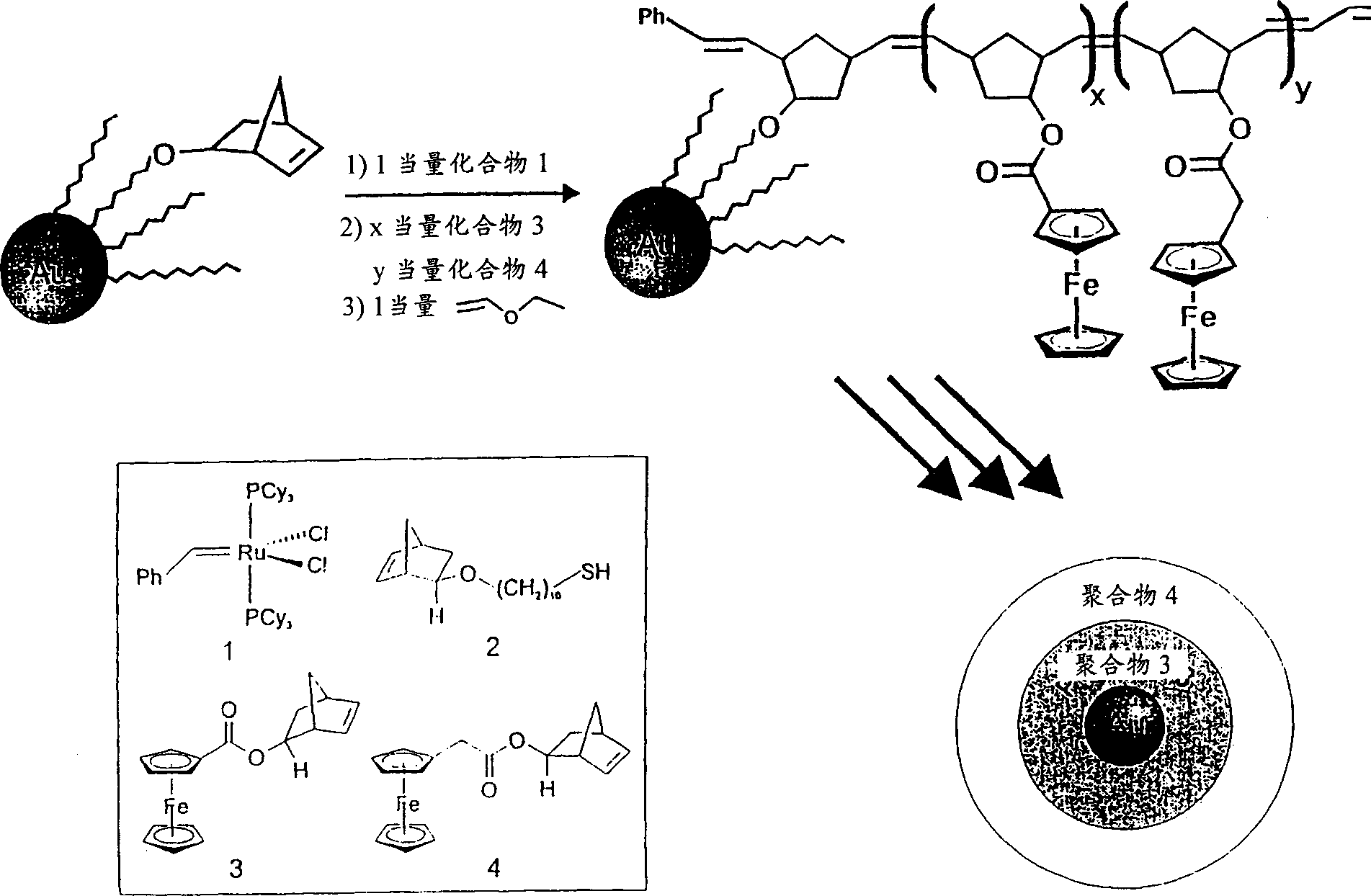
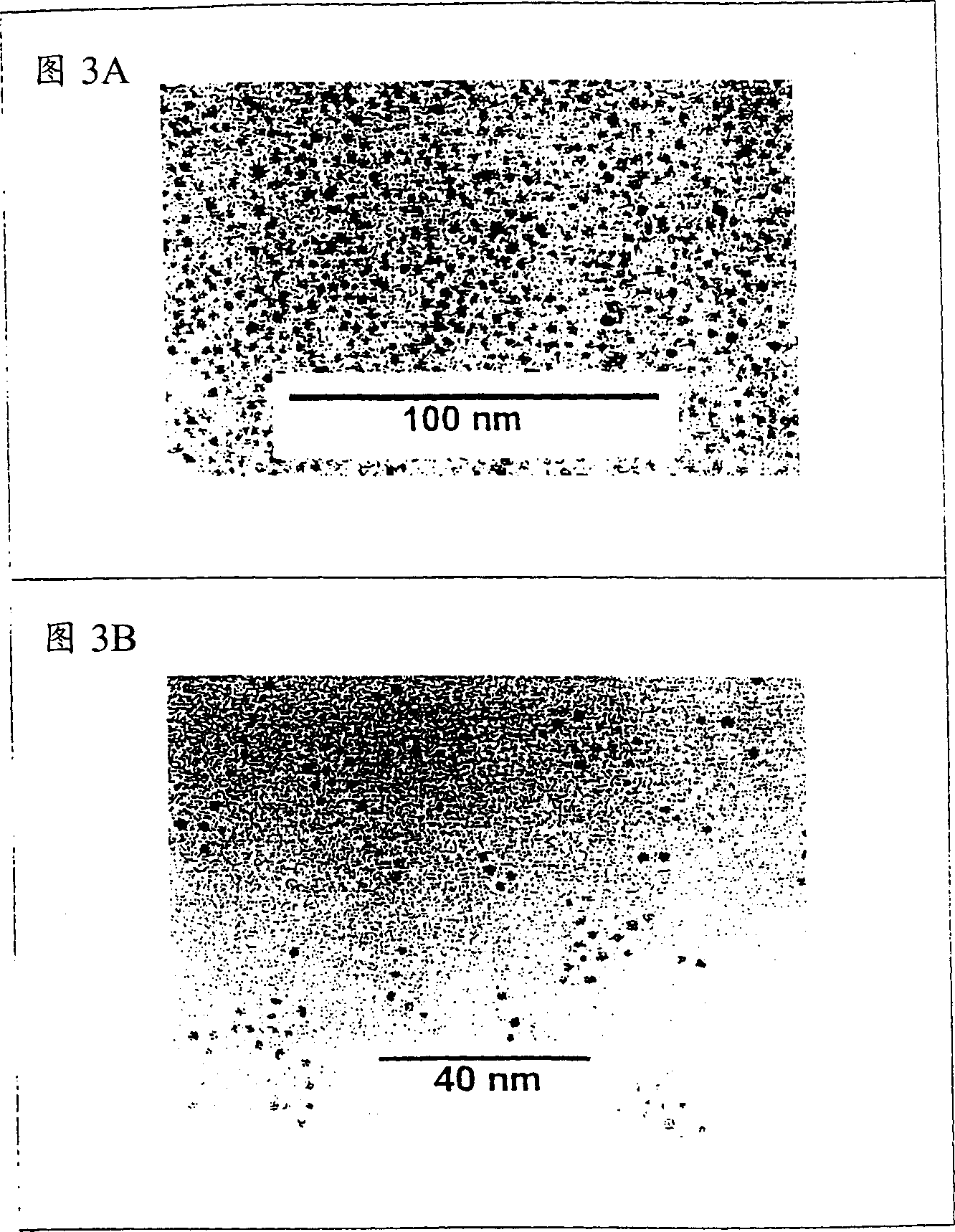
[0096] This example describes the preparation of novel metal-organic hybrid nanoparticles by, for example, figure 1 Ring-opening metathesis polymerization (ROMP), as described in , controls polymer growth on the surface of gold nanoparticle templates. In this method, organosoluble gold nanoparticles (GNPs) are modified with norbornene-terminated linear alkanethiols (2). Then, the ROMP catalyst (1) capable of enduring functional groups is used to directly initiate the polymerization reaction on the surface of the particles, and then the monomer raw material containing norbornene groups is injected into the solution of the initial nanoparticles.
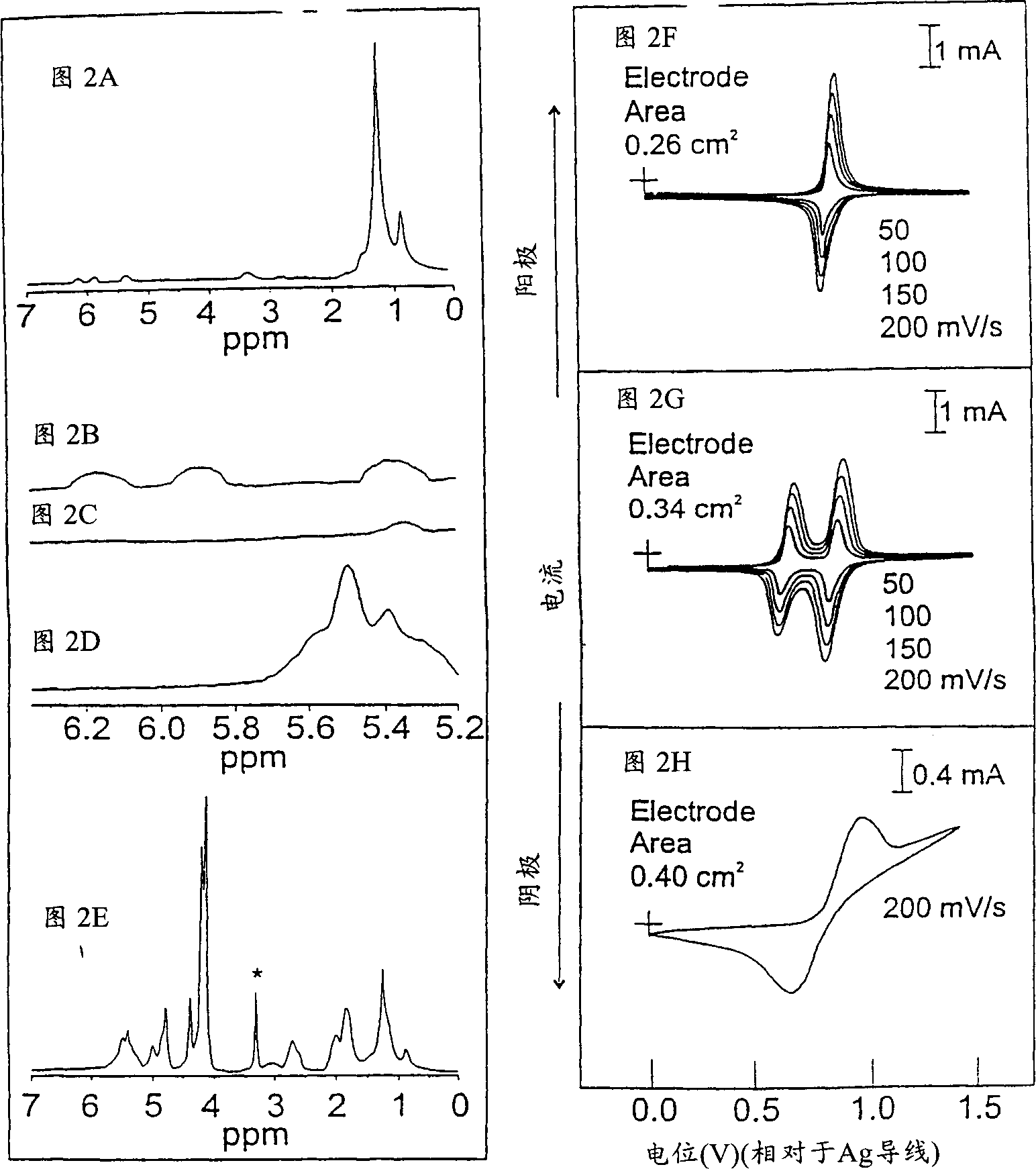
[0097] Two tentative conceptual systems emerged. The first is GNPs with a polymer shell of ferrocene-3 functionalized with norbornene groups with redox activity. The second is GNPs functionalized with the initial unit of 3 and subsequently functionalized with another redox-active norbornenyl-containing monomer 4. The redox potential...
Embodiment 2
[0150] This example describes compound 5 (see Figure 4 )Synthesis. To a 100 mL Schlenk flask was added 2-norbornene-5-exo-ol (1.10 g, 10 mmol), 3-thiopheneacetic acid (1.42 g, 10 mmol) and p-toluenesulfonic acid monohydrate (80 mg, 0.42 mmol). The above three solids were dissolved in toluene (60 mL) and a Dean / Stark trap was fitted on top of the flask. A water condenser was placed on top of the Dean / Stark trap and the mixture was heated to reflux. Over 6 hours, the volume of the reaction mixture was reduced to 20 mL by occasionally collecting solvent from the bottom of the Dean / Stark trap. The mixture was cooled to room temperature, poured into water (50 mL), and extracted with ether (3 x 50 mL). The organic portions were combined, washed with brine (50 mL), dried over sodium sulfate, and filtered into a 500 mL round bottom flask. The solvent was removed under vacuum with a rotary evaporator. The resulting pale yellow oil was chromatographed on silica gel with 1:1 CH 2 ...
Embodiment 3
[0158] This example describes compound 6 (see Figure 4 )Synthesis. In an inert atmosphere glove box, exo-5-norbornen-2-ol (710 mg, 6.45 mmol) was weighed and placed in a 50 mL Schlenk flask. THF (15 mL) was added, and while the solution was vigorously stirred, sodium metal (160 mg, 6.96 mmol) was added free of oil. The mixture was then removed from the glove box, refluxed under nitrogen sparging for 12 hours, and allowed to cool to room temperature. In a separate 100 mL Schlenk flask, 2,5-dibromo-3-bromomethylthiophene (2.01 g, 6.00 mmol) was dissolved in THF (15 mL), and the flask was fitted with an isobaric dropping funnel. The cooled deprotonated exo-5-norbornen-2-ol solution was then transferred via cannula to an isobaric dropping funnel (excess Na was quenched with isopropanol) and, under vigorous stirring, Add slowly to the thiophene solution over 10 minutes. Afterwards, the dropping funnel was replaced with a condenser, and the mixture was refluxed for another 12 h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com