Intravaginal clindamycin ovule composition
A technology of clindamycin and drug clindamycin, applied in the directions of suppository delivery, pharmaceutical formulation, oil/fat/wax inactive ingredients, etc., can solve the problem of inconvenience of vaginal cream
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Preparation of suppository
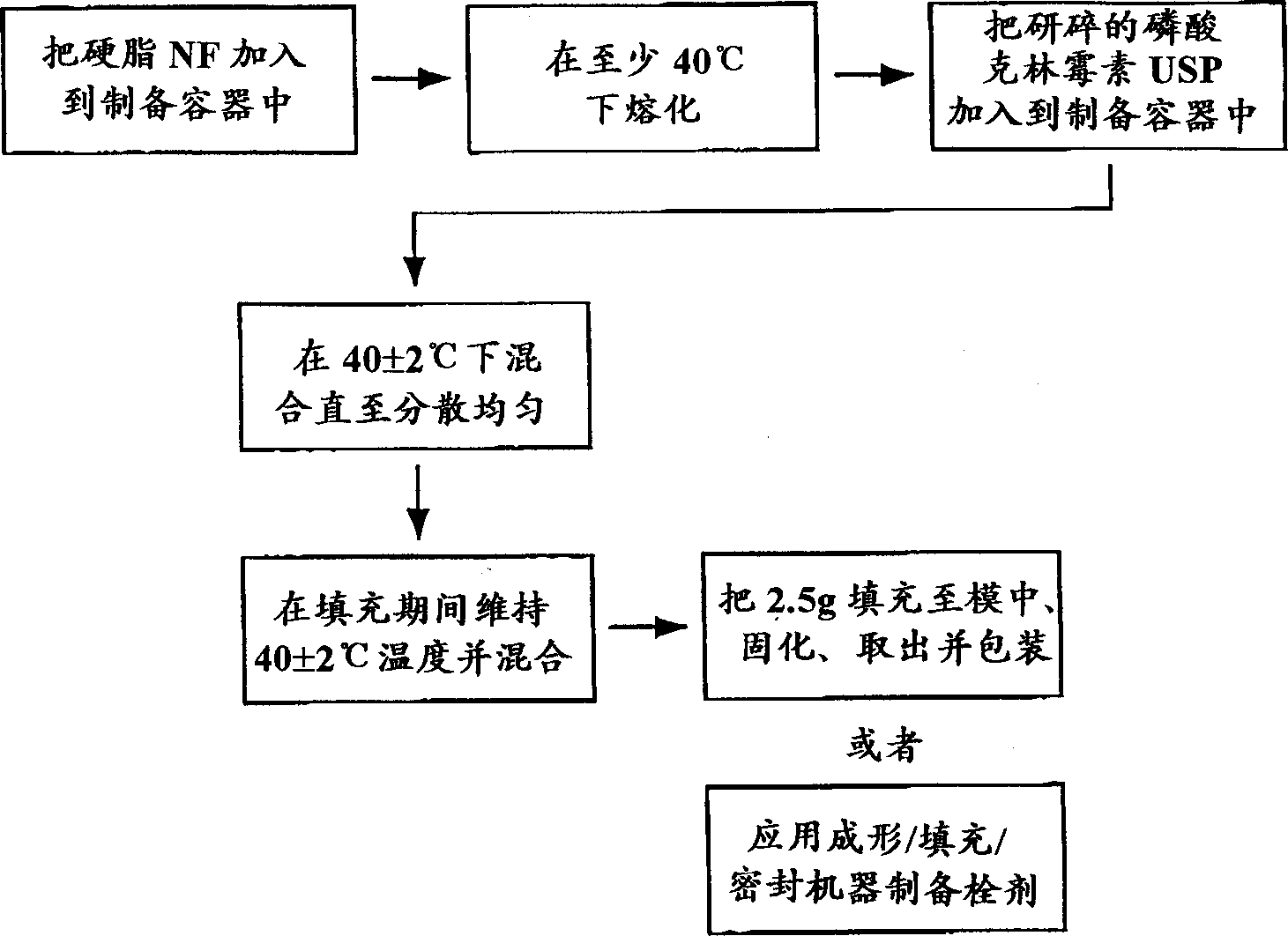
[0040] The following method was used to prepare a batch of 11,200 suppositories.
[0041] 1. In the preparation tank, 29kg WITEPSOL H-32 stearin NF matrix is heated to 40±2℃ to melt. During the whole preparation process, the temperature of melting the suppository base was maintained at 40±2°C.
[0042] 2. Using a preheated filter, transfer 26.614 kg of molten matrix to a second preparation vessel equipped with a homogenizing mixer.
[0043] 3. Add 1.386 g of clindamycin phosphate equivalent to 1.12 kg of clindamycin free alkali into the above tank, mix and homogenize them to obtain a uniform dispersion.
[0044] 4. Transfer the drug dispersion to the jacketed tank and transport it to the forming / filling / sealing suppository machine.
[0045] 5. Maintain mixing and maintain the temperature at 40±2°C. Use an automatic forming / filling / seal machine to shape the drug dispersion into a 2.5g suppository.
Embodiment 2X
[0046] Example 2 X-ray diffraction detection
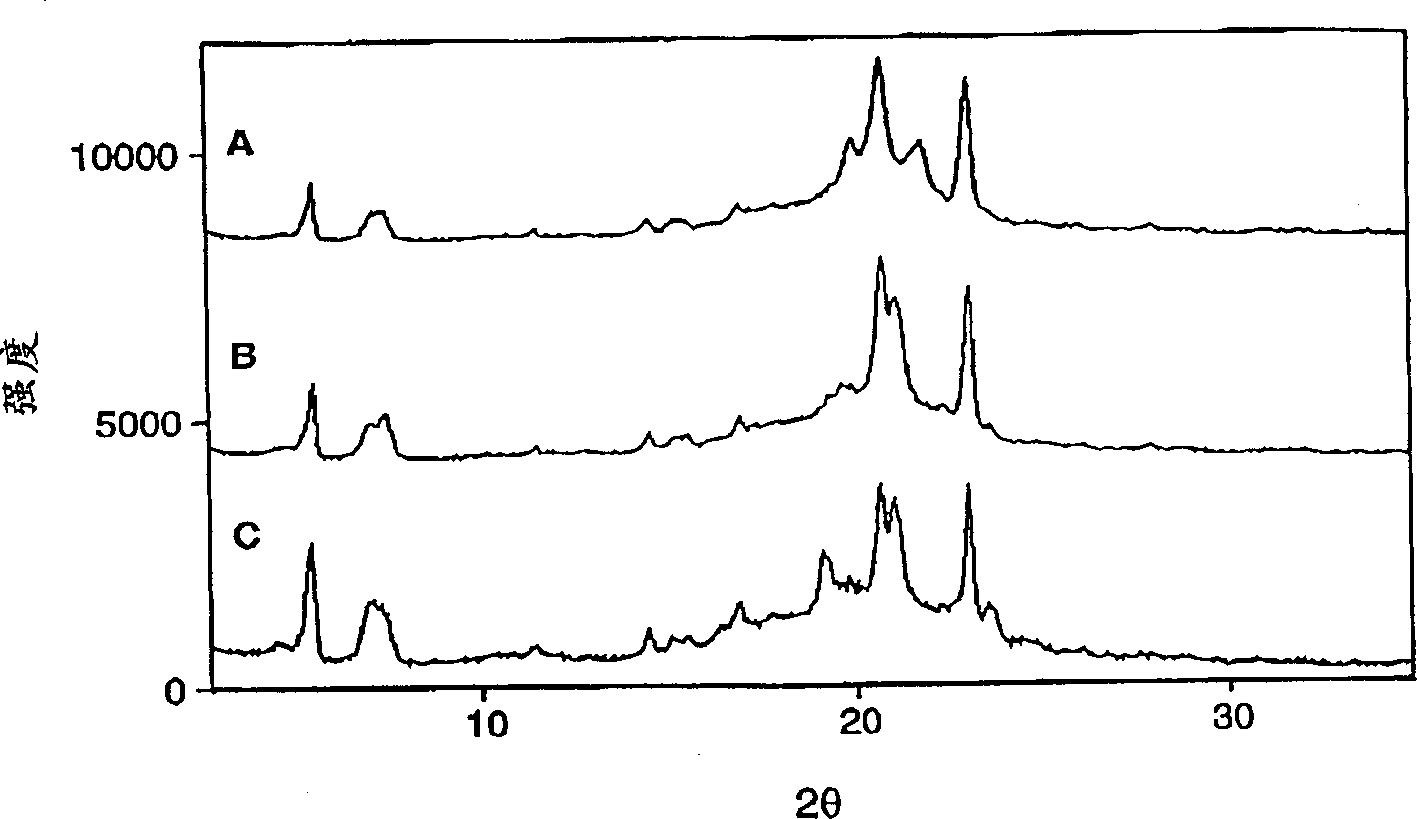
[0047] The Siemans D-5000 x-ray diffractometer was used to determine the polymorphic transformation state of the suppository. Scrape a sufficient amount of material to fill the sample pan of the diffractometer from the suppository, and then carefully fill it into the pan to ensure a flat surface. Pass the copper K-L with a nickel filter at a wavelength of 1.5406 3 Ray operation instrument. The instrument parameters are as follows: 45KV voltage, 40mA current, 0.2mm detector hole. Scan the sample at an angle of 3-40° 2θ at 2° 2θ / min. Figure 1 shows a typical diffraction pattern of a polymorphic phase transition sample from α to α'to β.
Embodiment 3
[0048] Example 3 Flow point determination
[0049] The flow point of the suppository was measured in the following way: A polarizing microscope equipped with a 20×pol long working distance objective lens was used in combination with a Mettler FP82 hot stage. Use a razor blade to obtain a small amount of suppository, place it on a pre-cleaned glass slide, and cover it with a cover glass. Use a light press to cover the cover glass to spread the sample evenly, and place the glass slide in the hot stage furnace. The sample is then heated in the range of 25-40°C at a rate of 2°C / min. Use a camera to observe the heating process, and use a synchronized screen digital temperature display and record. The pour point is defined as the temperature at which rapid flow of the sample occurs.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com