Process for preparation of pure citalopram
A technology of citalopram and citalopram base, which is applied in the fields of nervous system diseases, organic chemistry, and drug combination, and can solve problems such as cumbersome, expensive purification processing, and difficult removal of impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
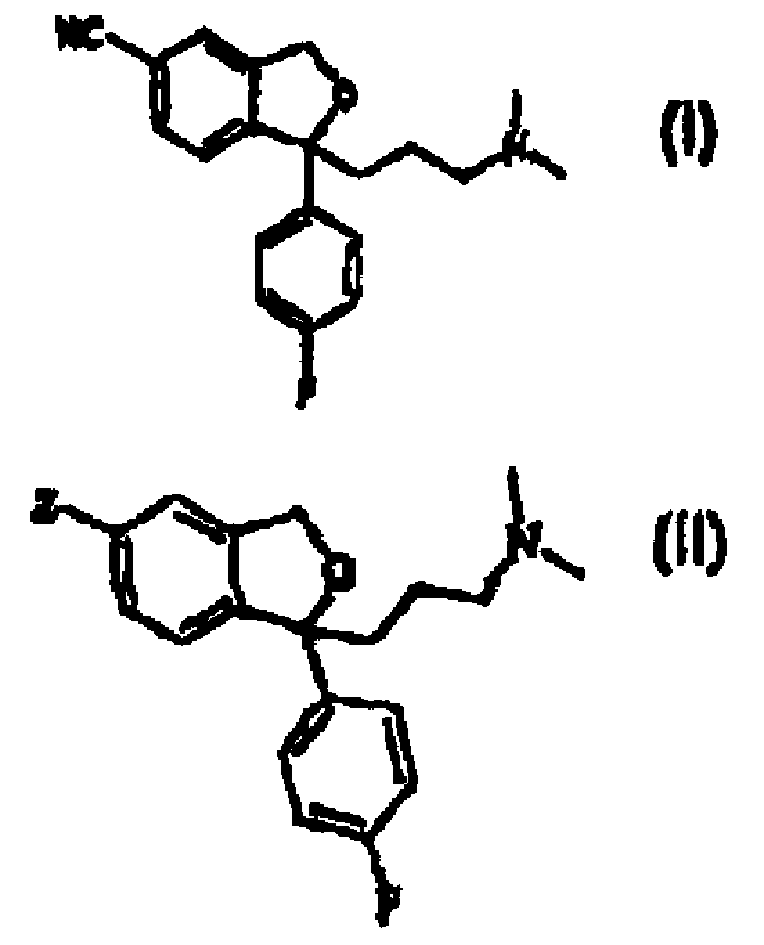
[0054] Preparation of crude citalopram base (5-cyano-1-(3-dimethylaminopropyl)-1-(4-fluorophenyl)phthalane)
[0055] Cu(I)CN (197g, 2.2mol) was added to 5-bromo-1-(4-fluorophenyl)-1-(3-methylaminopropyl)phthalane (720g, 1.9mol) in sulfolane (250mL) in the solution. After the reaction mixture was heated at 150 °C for 5 h, sulfolane (500 mL) was added. The reaction mixture was cooled to 80°C, at which point ethylenediamine (aq, 50% w / v) was added. Toluene (2 L) was added and phase separation occurred. The organic phase was further washed with EDTA (500 mL 5% w / v in water) and water (2 x 500 mL). Volatile materials from the organic phase were removed under vacuum. 540 g of crude citalopram base were isolated as an oil. Purity about 85%, determined by HPLC (peak area).
example 2
[0057] Purification of crude citalopram by thin film distillation
[0058] Crude citalopram base (5-cyano-1-(3-dimethylaminopropyl)-1-(4-fluorophenyl)phthalane) (20kg, purity about 89%, according to HPLC (peak area) and sulfolane (4L) heated to about 100 ° C. The hot mixture is fed into thin film distillation equipment (wiped film distillation), where the scraper temperature is 245 ° C, resulting in a pressure of about 0.7 mm Hg. The temperature of the effluent after condensation is maintained at 120° C. to prevent crystallization of the free base. The distillate contained crude citalopram (purity about 96% by HPLC (peak area)) and sulfolane.
example 3
[0060] The above distillate (crude citalopram base) is further purified by crystallization of citalopram free base
[0061] The distillate (4 kg) was dissolved in methanol (12 L) at ambient temperature as described above. Water is added until the mixture maintains a "creamy" color. The mixture was seeded with crystals of citalopram free base. When the temperature was lowered to 10° C. for 2 h, the crystals were separated by filtration. The crystallization from methanol / water as described above was repeated. Two further recrystallizations from n-heptane yielded citalopram (2.3 kg) free base with a purity of about 99.5% (HPLC: peak area).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com