Medicine releasing system of aliphatic polylactone mixture
A polylactone and aliphatic technology, applied in the field of drug release system, can solve the problems of poor drug permeability, slow degradation rate, and inability to achieve constant drug release.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
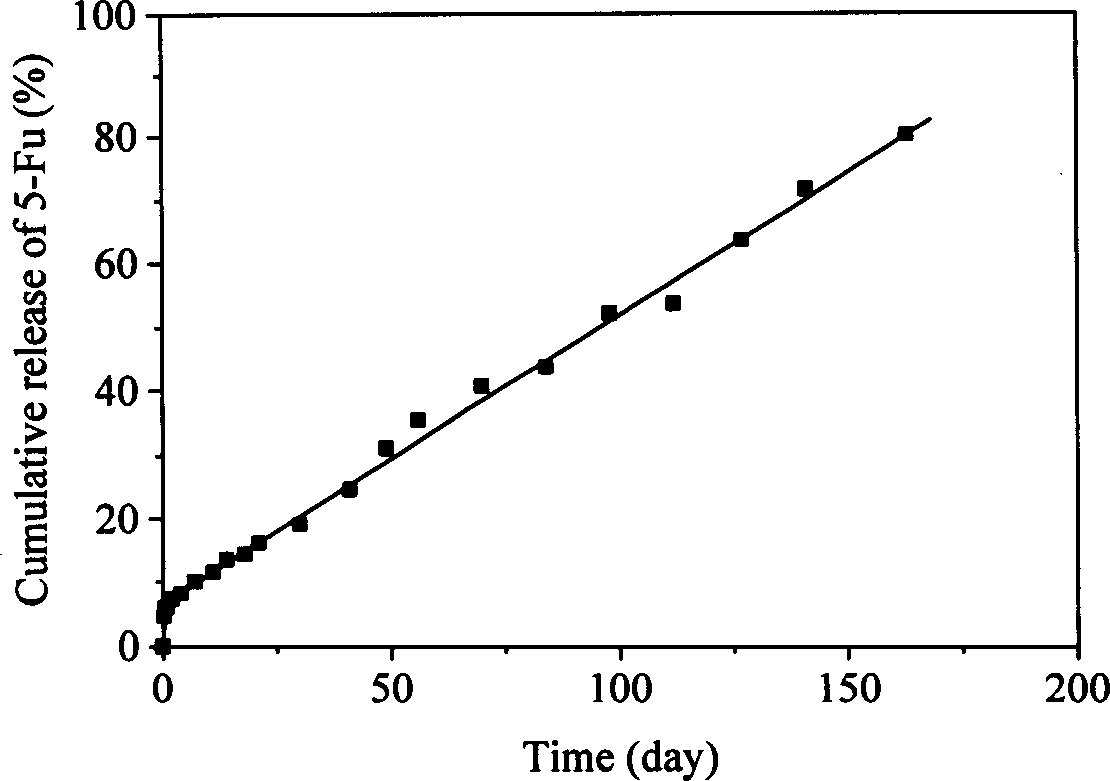
[0018] The molecular weight is 1×10 3 , 2×10 3 , 5×10 3 , 8×10 3 , 1.7×10 4 , 4.9×10 4 , 1×10 5 , 2.1×10 5 and 3.3×10 5 After dissolving 0.1g of each of the nine polylactide homopolymers with different molecular weights in 20ml of dichloromethane, add 0.1g of 5-fluorouracil (5-Fu), and make it uniformly dispersed in the solution by grinding, and wait for the solvent to volatilize After completion, a 5-Fu tablet with a diameter of 10 mm and a thickness of about 1 mm was prepared by molding at 180° C. with a mold. The tablet was subjected to an in vitro drug release test in a phosphate buffer solution of pH 7.4 at 37° C., and the amount of drug released was detected by an ultraviolet spectrophotometer (λ=266nm). The pharmaceutical preparation has no initial stagnation period in the release process, and the initial burst release is very small, and the constant release time is six months.
Embodiment 2
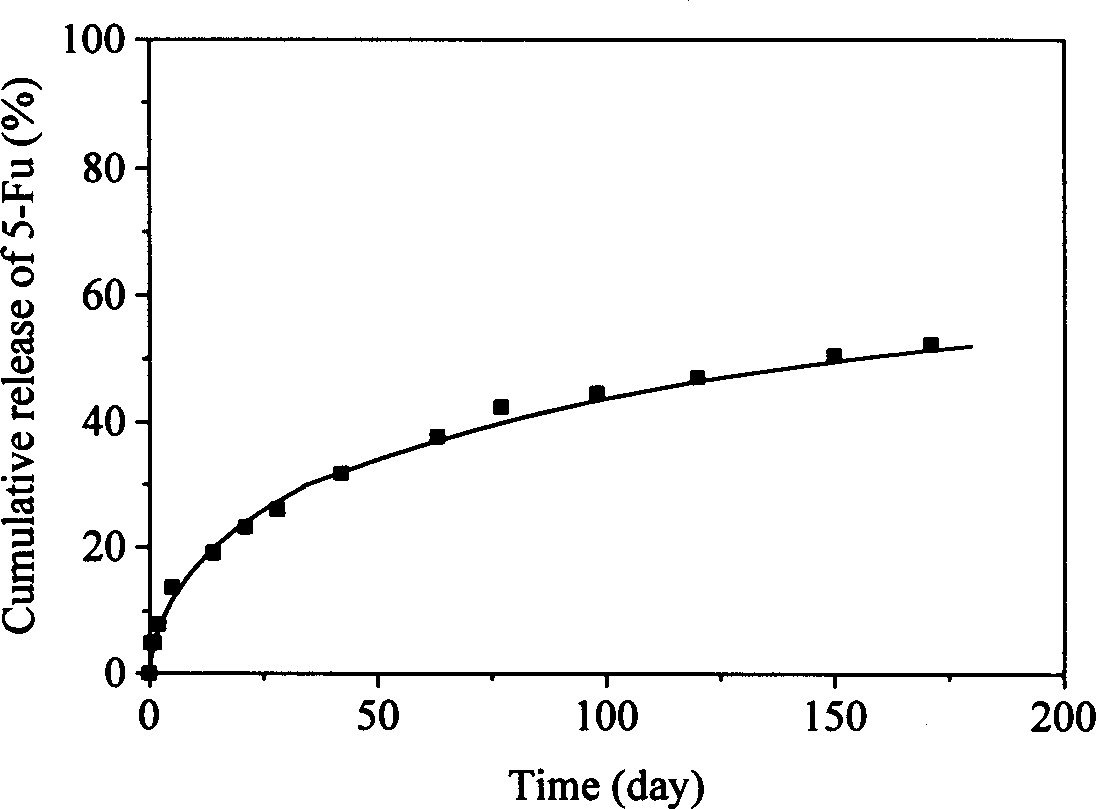
[0020] 7g has a molecular weight of 2.1×10 4 3 g of polylactide and a molecular weight of 1.7 x 10 4 After the polycaprolactone homopolymer was dissolved in 200ml of dichloromethane, 1g of 5-fluorouracil (5-Fu) was added, and it was evenly dispersed in the above solution by grinding. ℃Prepare 5-Fu tablets with a diameter of 10mm and a thickness of about 1mm by compression molding. The tablet was subjected to an in vitro drug release test in a phosphate buffer solution of pH 7.4 at 37° C., and the amount of drug released was detected by an ultraviolet spectrophotometer (λ=266nm). Although the pharmaceutical preparation has a relatively obvious initial burst release (about 10%) during the release process, the sustained release time at a constant rate can still be maintained for more than one month.
Embodiment 3
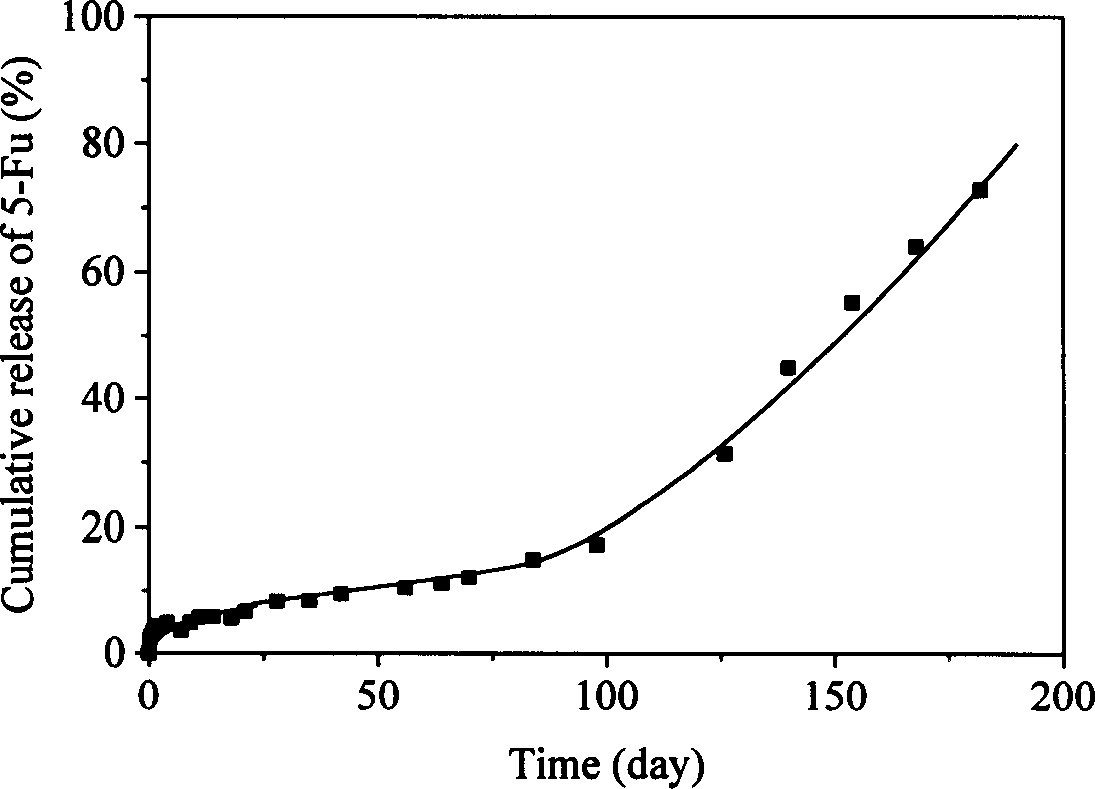
[0022] 0.5g molecular weight is 1.9×10 4 0.3 g of polylactide and a molecular weight of 5.6 x 10 4 After the polyhydroxybutyrate homopolymer was dissolved in 20ml of dichloromethane, 0.1g of 5-fluorouracil (5-Fu) was added, and it was uniformly dispersed in the solution by grinding. After the solvent was completely evaporated, a 5-Fu tablet with a diameter of 10 mm and a thickness of about 1 mm was prepared by molding at 180° C. with a mold. The tablet was subjected to an in vitro drug release test in a phosphate buffer solution of pH 7.4 at 37° C., and the amount of drug released was detected by an ultraviolet spectrophotometer (λ=266nm). This pharmaceutical preparation has no obvious initial burst release during the release process, and the sustained release time at a constant rate is as long as twelve months.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com