ECL (electrochemiluminescence) immunosensor for detecting tumor markers and preparation method and applications thereof
A tumor marker, luminescent immune technology, applied in the field of electrochemiluminescence immunosensor and its detection, to achieve the effects of accurate results, flexible and diverse methods, and strong selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
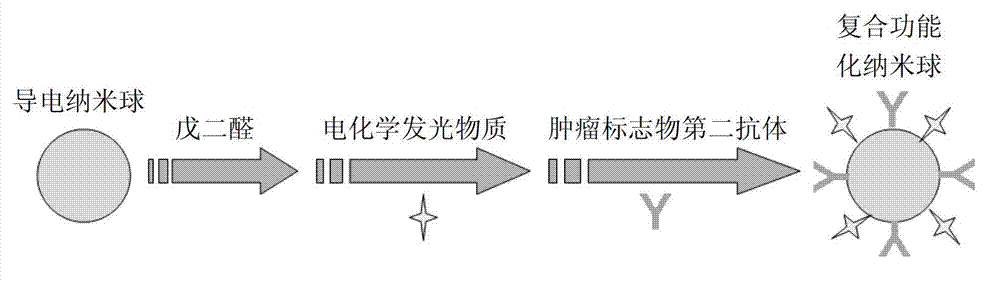
[0053] The synthesis of the composite functionalized nanospheres simultaneously labeled with the second antibody of the tumor marker and the electrochemiluminescent marker, the specific steps are as follows:
[0054] (a) Synthesis of conductive nanospheres: Add 90–100 mL of a mixture of ethanol, water, and concentrated ammonia into a clean beaker (V 乙醇 :V 水 :V 浓氨水 = 87:7:1), magnetically stirred for 30 min; after the solution was evenly mixed, slowly added 5 mL of tetraethyl orthosilicate (TEOS) dropwise while stirring magnetically; after the dropwise addition, sealed with parafilm The mouth of the beaker was reacted for 10 h; centrifuged at 4000 rpm, and then the precipitate was dispersed in ultrapure water to form a 10 mg / mL first suspension; 2 mL of the first suspension was put into a centrifuge tube, and 0.18 mL of 3- After aminopropyltriethoxysilane (APTES), mix and stir at room temperature for 7 h; centrifuge and wash at 4000 rpm, and then disperse the precipitate in ...
specific Embodiment 2
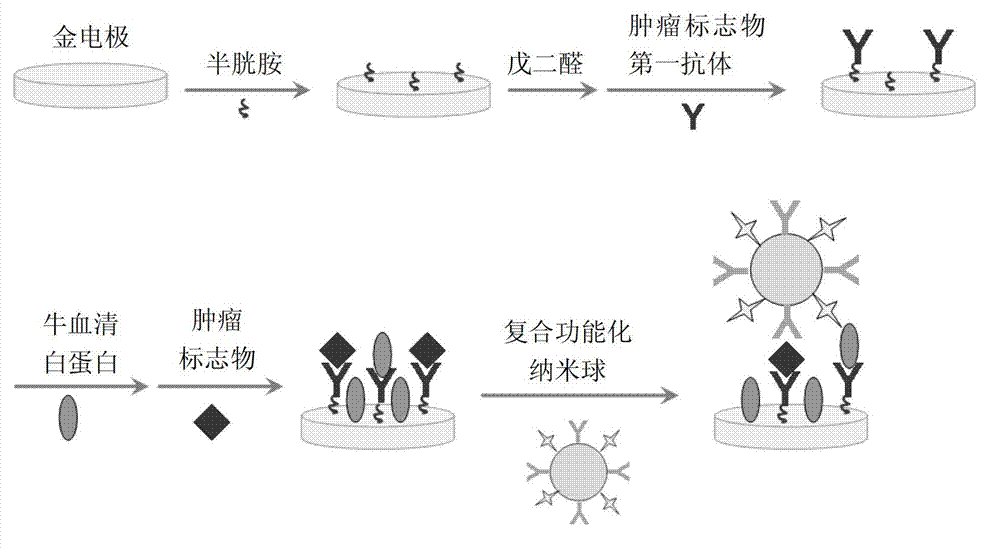
[0059] The preparation of the gold electrode assembled by cysteamine, its specific steps are as follows:
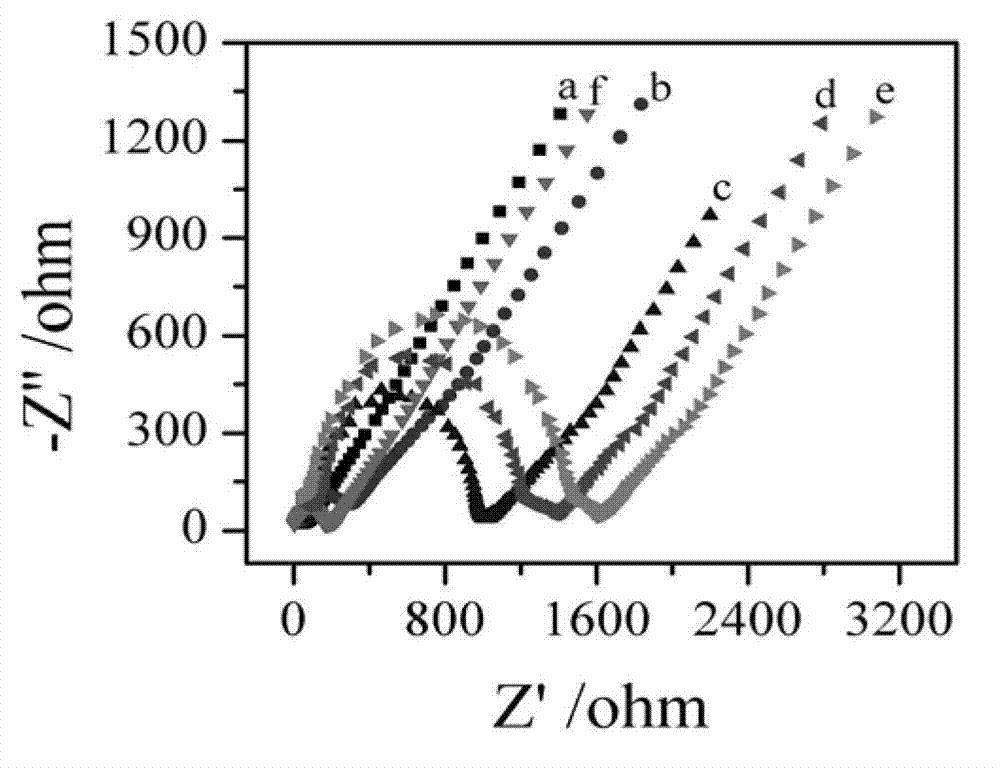
[0060] A gold electrode with a diameter of 3-5 mm was polished with 1.0 μm, 0.3 μm, and 0.05 μm Al2O3 polishing powder in sequence, and the gold electrode was rinsed with ultrapure water, ultrasonicated in ultrapure water for 2 min, and then placed in 0.5 M H 2 SO 4 In the process, the cyclic voltammetry scan was carried out in the potential range of 0-1.6 V, and the scan rate was 100 mV / s until the cyclic voltammetry curve was stable; after the gold electrode was rinsed with ultrapure water, soaked in 0.1 mol / L The cysteamine-modified gold electrode was obtained by reacting in the cysteamine solution at 4 °C for 10 h, and then rinsing with ultrapure water. This process can be monitored by AC impedance method. Such as image 3 As shown, the impedance of the bare electrode before unmodified cysteamine is very small (curve a), and the impedance of the gold electrode aft...
specific Embodiment 3
[0061] The preparation of the gold electrode assembled with glutaraldehyde and tumor marker primary antibody, its specific steps are as follows:
[0062] After rinsing the gold electrode in Example 2 with ultrapure water, soak it in an aqueous solution containing 2.5% glutaraldehyde at 4°C for 1 h; after rinsing the gold electrode with ultrapure water, soak it in 50 μg / mL of the tumor marker primary antibody was reacted at 4 °C for 12-18 h, rinsed with ultrapure water, and the gold electrode modified with glutaraldehyde and tumor marker primary antibody was obtained. This process can be monitored by AC impedance method. Such as image 3 As shown, the impedance of the gold electrode after modification of the glutaraldehyde and the primary antibody of the tumor marker increased greatly (curve c), indicating that the glutaraldehyde and the primary antibody of the tumor marker were successfully assembled on the gold electrode.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com