Method for producing medicine using silkworm expressed numan epidermal growth factor
A technology of epidermal growth factor and medicine, applied in the field of expressing human epidermal growth factor to produce medicine, can solve the problem of high production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] According to the reported EGF gene sequence (Proc.Natl.Acad.Sci.USA, 1983, VOL80,7462), synthesize 4 oligonucleotide chains of encoding EGF with DNA synthesizer and phosphite triester method, use poly Separation and purification by acrylamide-7mol / L urea denaturing gel electrophoresis, the sequences are: oligonucleotide chain 1: 5'-GGA ATT CGT TAA CTC CGA CTC CGA ATG TCC ATT GTCCCA CGA CGG TTA CTG TTT GCA CGA-3 ', oligonucleotide strand 2: 5'-AAGCTT TGG ACA AGT ACG CCT GTA ACT GTG TTG TTG GTT ACATCG GTG AAA GAT GTC AAT A-3, oligonucleotide strand 3: 5'-GGA ATT CTCATT ATT CCC ACC ACT TCA AGT CTC TGT ATT GA ATC TTT CACCGA TGT-3, oligonucleotide strand 4: 5'-GGC GTA CTT GTC CAA AGC TTCGAT GTA CAT ACA AAC ACC GTC GTG CAA ACA GTA ACCGTC-3', oligonucleotide Phosphorylation of the 5' end of the polynucleotide chain, renaturation, chain extension, ligation, EcoRI endonuclease digestion, and cloned into the EcoRI (Boringman Company) site of the pBluscript SK (CLONTECH Company) p...
Embodiment 2
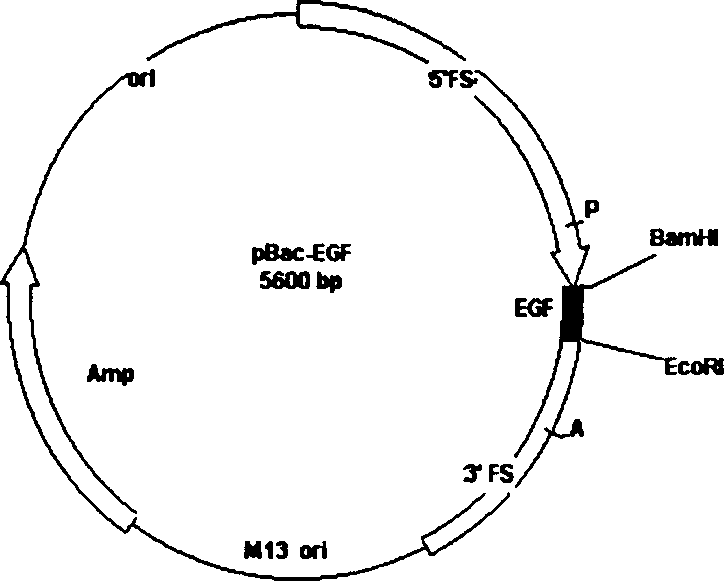
[0014] After the plasmid pSK-EGF containing the human EGF gene fragment was digested by BamHI (Boringman Company) and EcoRV, the gene fragment was recovered with low-melting point glue, and transferred with the transfer vector pBacPAK8 (CLONTECH Company) double-digested by BamHI and SmaI. connection to obtain the recombinant transfer vector plasmid pBacEGF ( figure 1 ), the gene was identified to be correct by restriction analysis. Embodiment 3, the acquisition of the recombinant baculovirus of human EGF gene
Embodiment 3
[0015]Get 5 ul of the silkworm baculovirus transfer plasmid pBacEGF containing human EGF gene and 6 ul of the modified virus BmBacPAK6 (constructed by the applicant) through AocI (Boringman Company) enzyme digestion and linearization, and add 100 ul of serum-free TC-100 (GIBCOBRL Company ) culture medium was mixed evenly. Take 6ul Dosper (Bowringman Company) and add 100ul serum-free TC-100 medium and mix well. Wash the Bm N cells previously cultured in a 35mm dish (preserved strain of the Institute of Biochemistry, Zhejiang University) twice with serum-free TC-100 medium, and add the transfer plasmid and Dosper mixture dropwise, and incubate at 27°C for 4-5 On the next day, the supernatant was collected for the first round of plaque screening. Take 5 ul supernatant to infect silkworm cells in a 35mm plate, discard the supernatant after 1 hour and add an equal amount of mixed TC-100 medium and low melting point agarose. Pick plaques after 4-5 days, infect silkworm cells for 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com