Conditional duplicated adenovirus and its establishment and use
An adenovirus, replication-type technology, applied in the field of biomedicine, can solve problems such as not particularly strict control, virus replication, and limited application prospects of viruses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Isolation of Tumor-Specific Promoter A. Telomerase 5' Regulatory Sequence by PCR Reaction and Cloning
[0022] During natural DNA replication, the ends of chromosomes shorten with each cell division. The integrity of telomeres at the end of chromosomes is crucial to the stability of chromosomes. The integrity of telomeres is maintained by telomerase. Telomerase is inactive in more than 99% of normal cells and is reactivated in more than 90% of tumor cells. Experiments have shown that the activity of telomerase is controlled by its promoter. The promoter of telomerase (TERT) is active in most tumors but not in normal cells. In this way, the promoter of TERT becomes a tumor-selectively activated promoter. In order to isolate the telomerase promoter, the present invention adopts PCR technology, uses human cell DNA as a template, amplifies the promoter sequence 1, inserts it in the plasmid pEGFP-1 (purchased from Clontech Company), drives GFP gene expression, constructs...
Embodiment 2
[0028] Construction of tumor-specific replication-competent adenoviral vectors
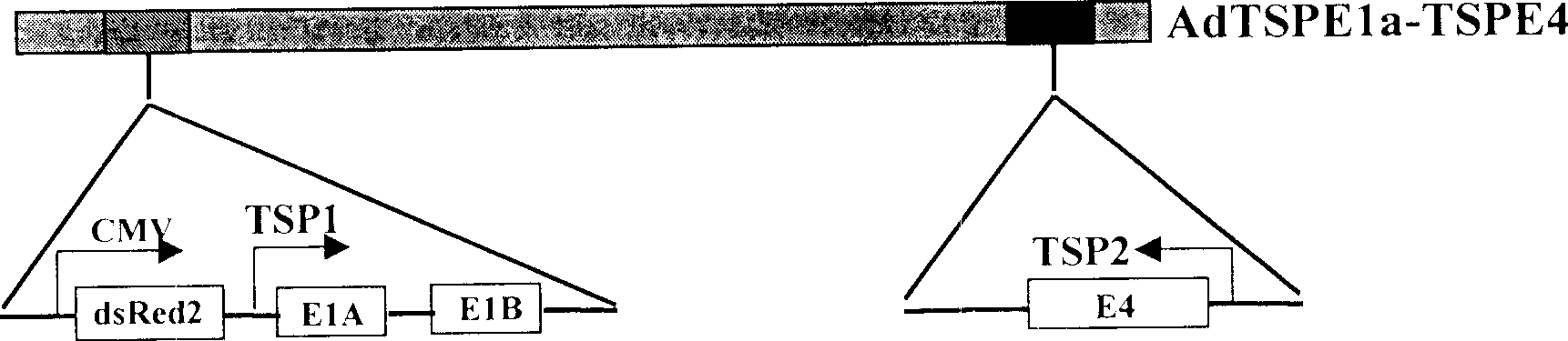
[0029] The tumor-specific promoter isolated in Example 1 was embedded into the DNA of the adenovirus by genetic engineering, and used to control the expression of early genes necessary for the replication of the adenovirus. figure 1 Described is the construction method of recombinant virus. The virus is characterized in that their E1A and E4 genes are simultaneously regulated by tumor tissue-specific promoters. The promoters controlling E1A and E4 may be the same promoter or different promoters. The following combinations are combinations of conditionally replicative adenoviruses employed in the present invention.
[0030] 1) AdHRPE1aTERTE4-dsRed2, TSP1 is a hypoxia-inducible promoter, and TSP2 is a telomerase promoter;
[0031] 2) AdAFPE1aTERTE4-dsRed2, TSP1 is the α-embryoprotein promoter, and TSP2 is the telomerase promoter;
[0032] 3) AdE2F1E1aTERTE4-dsRed2, TSP1 is E2F1 promoter, TSP2 is...
Embodiment 3
[0037] Packaging, amplification, purification of virus, and characterization of infection and replication
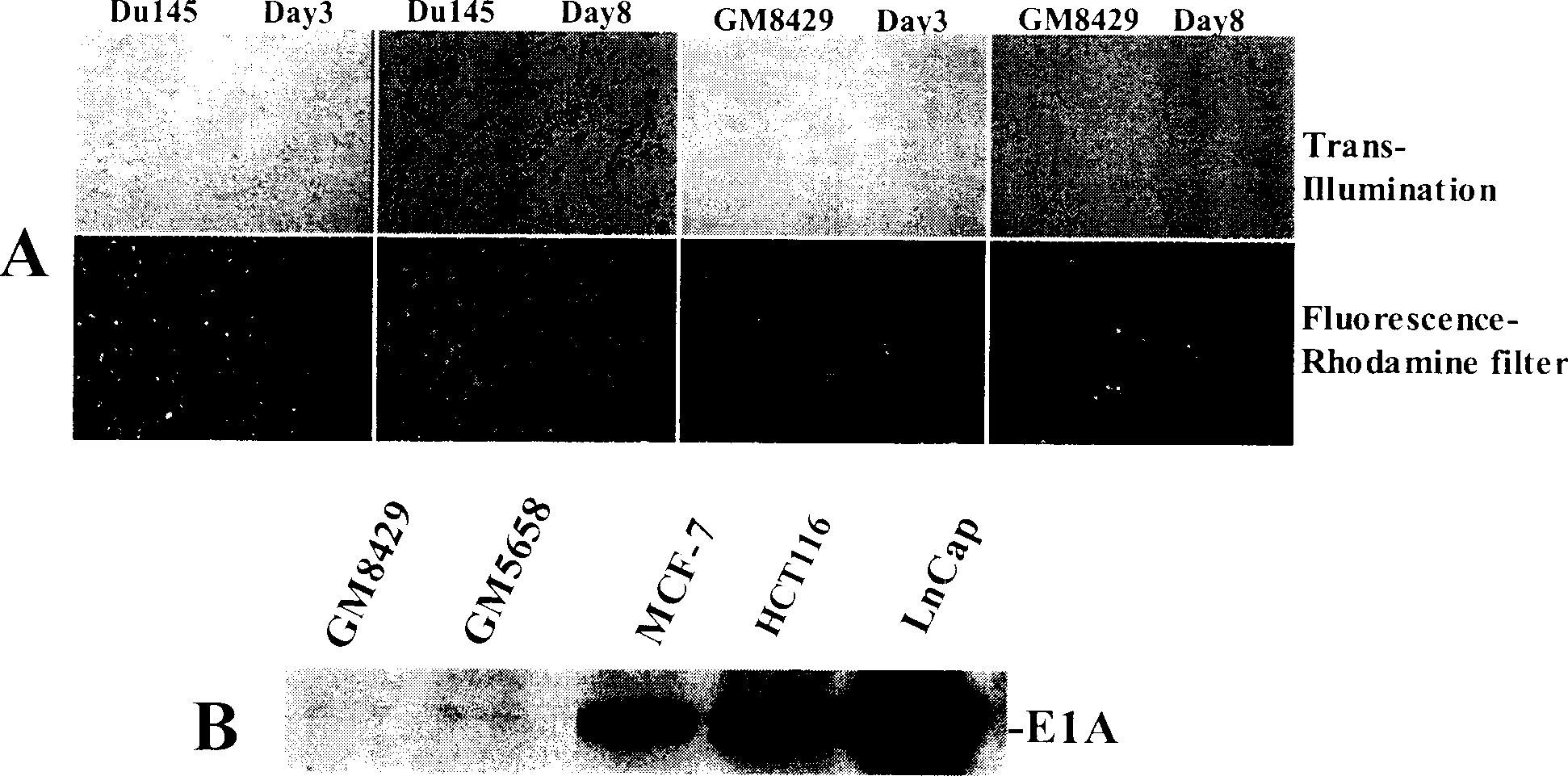
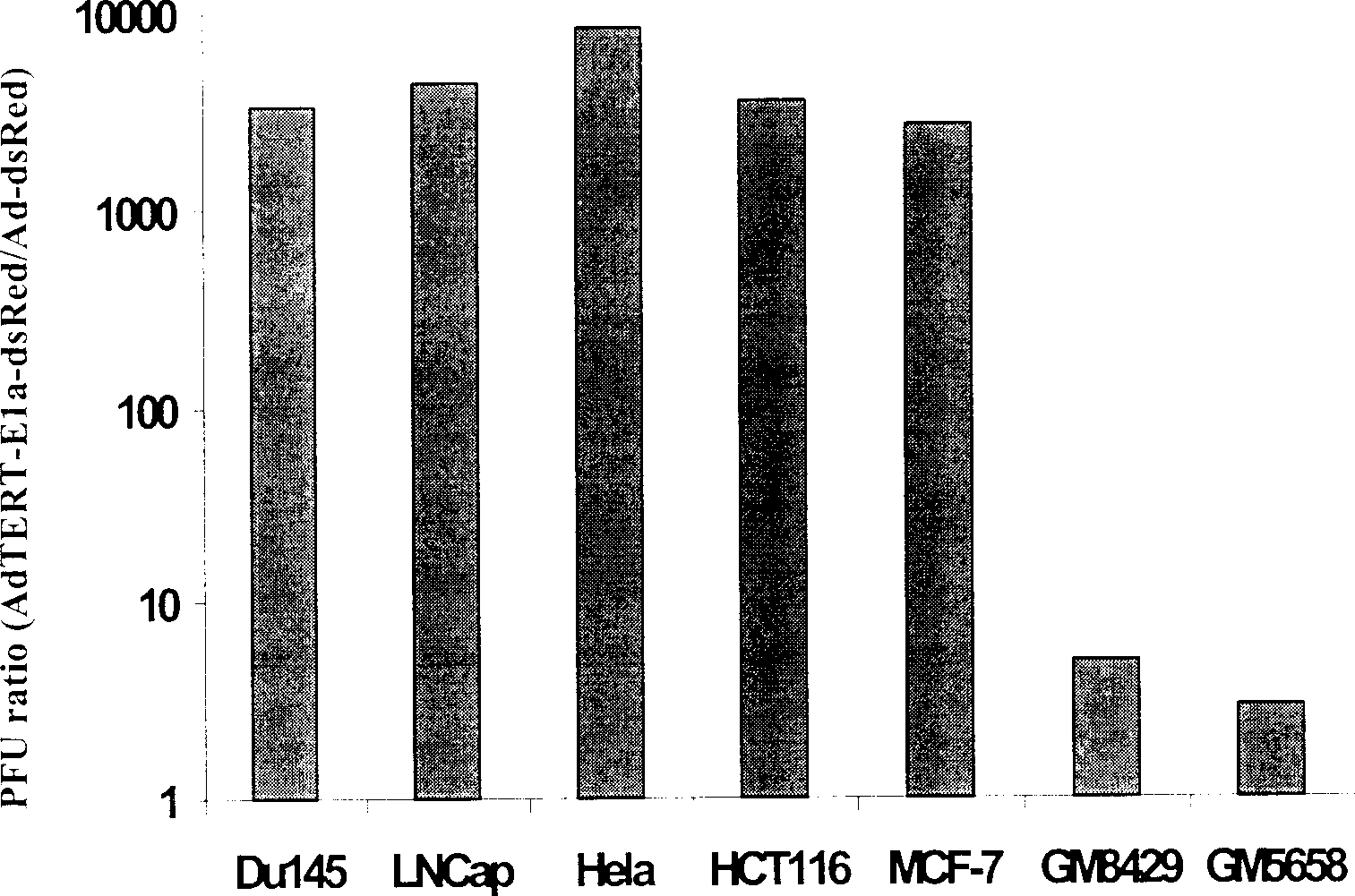
[0038] Human embryonic kidney 293 cells were used to package the virus constructed above, and then amplified after screening by plaque purification, and the virus was purified by cesium chloride density gradient centrifugation and dialysis. The titer of purified virus can reach 3~5×10 10 pfu / ml. Observe virus replication in various tumor and normal cells. The result is as follows:
[0039] 1) AdTERTE1a-E4-dsRed2 can infect the following tumor cells, such as breast cancer, prostate cancer, ovarian cancer, cervical cancer, colon cancer, etc., and replicate in large numbers in these cells. Obvious cytopathic changes appeared 3-5 days after infection, and then the cells lysed and continued to infect surrounding tumor cells, and almost all tumor cells were killed within 3-7 days. The virus can also infect human fibroblasts and normal epithelial cells, but does not replica...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com