Use of celecoxib composition for fast pain relief
A technology for relieving pain and composition, which is applied in the field of rapid pain relief of Selexib composition, which can solve the problems of not containing Selexib and unpredictable plasma concentration, etc., and achieve the effect of rapid pain relief
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
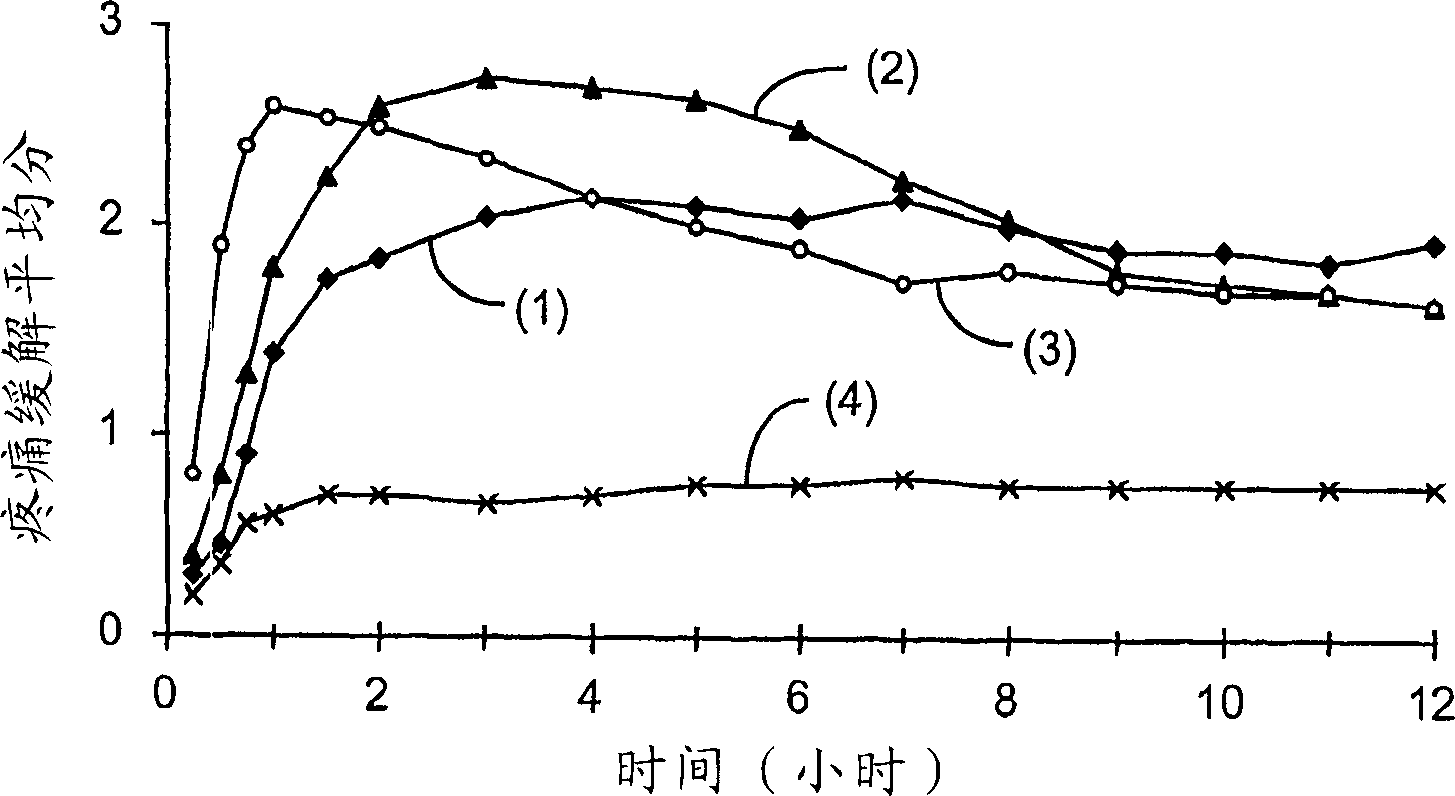
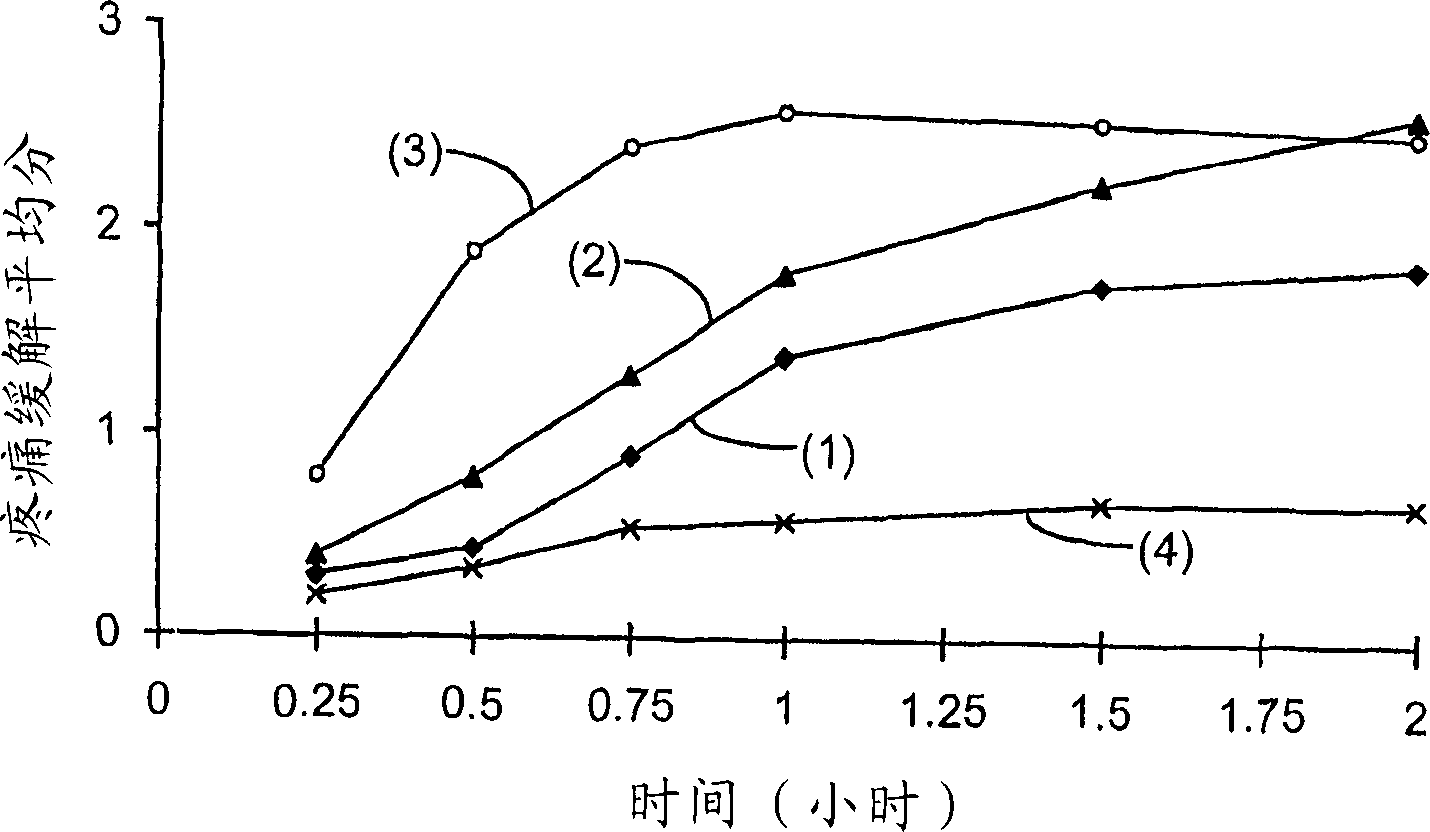
[0097] A single-center, single-dose, double-blind, placebo-controlled, parallel-group 24-hour study of 200 patients experiencing moderate to severe post-operative pain was studied. The patient is in the postoperative phase after removal of 2 or more third molars requiring bone resection. Patients were stratified according to baseline pain intensity and randomized into 4 treatment groups, administered orally at the following doses:
[0098] 1. 200mg of Selixi capsules (Celebrex 200mg).
[0099] 2. 400 mg of ibuprofen capsules.
[0100] 3. 200mg fine suspension of Selixix.
[0101] 4. Placebos.
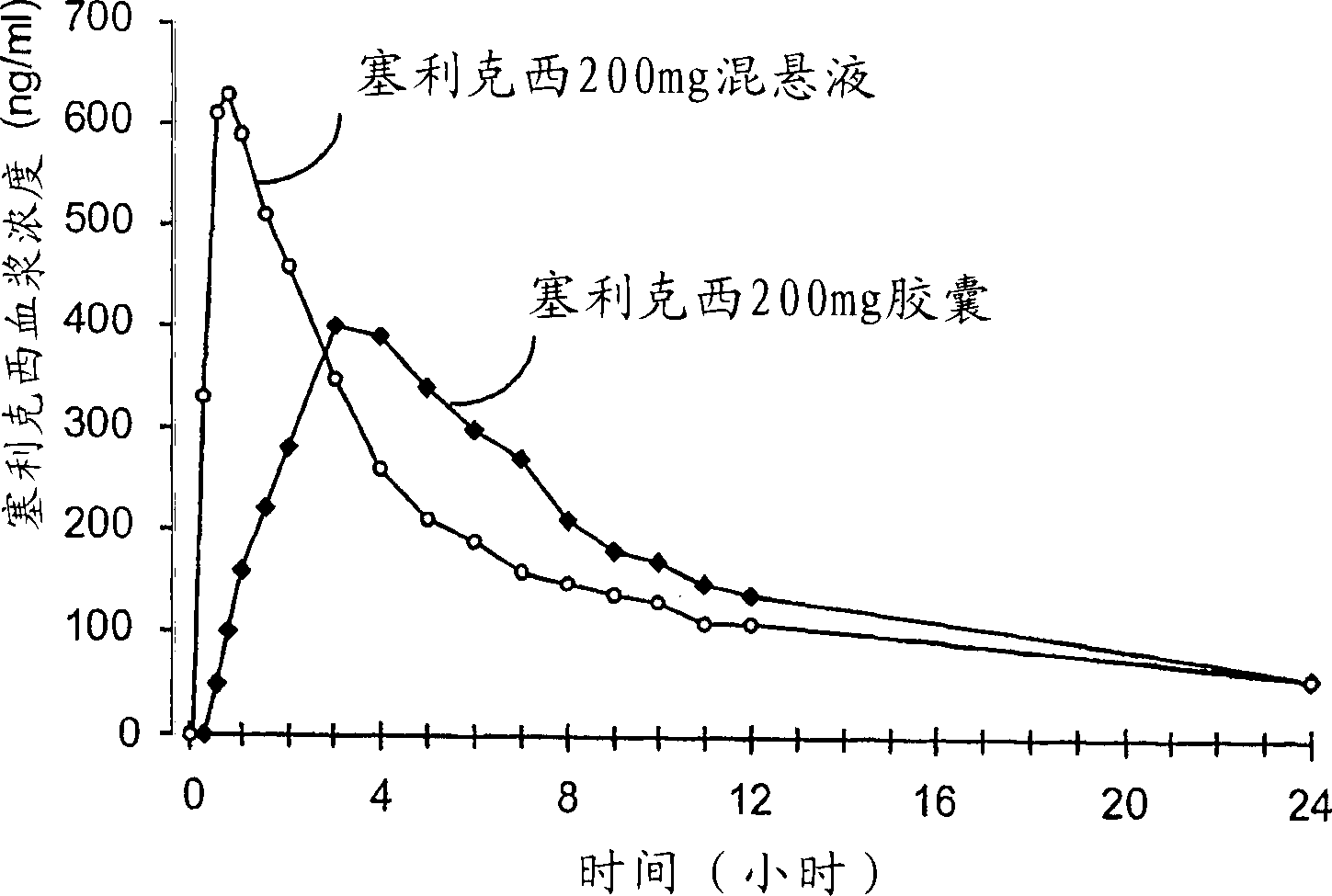
[0102] A fine suspension of Selixix was prepared by dissolving Selixix in ethanol containing 5% polysorbate 80 and adding the resulting mixture to apple juice. The fine suspension was administered within 5 minutes of preparation.
[0103] Individual patient assessments of pain relief on a patient visit basis were at the time of dosing and at 15, 30, 45 and 60 minutes after dosi...
Embodiment 2
[0114] 1 gram of the SF-1 formulation was individually filled into individual hard gelatin capsules (Capsugel) to form Test Composition 1.
[0115] For comparison, Selixix suspension was formulated as follows:
[0116] A. Add 5.0g Tween 80 into the volumetric flask.
[0117] B. Add ethanol (to 100ml) to form a mixture, and stir the mixture to form a homogeneous solution.
[0118] C. Transfer a 5 ml aliquot of this homogeneous solution to another flask containing 200 mg of Selexib to form a premix.
[0119] D. Add 75ml of apple juice to the premix to form the Selixix Suspension Intermediate.
[0120] E. The Selexib Suspension Intermediate was left to stand for 5 minutes and then shaken to form the Selexib Suspension for comparison.
[0121] In a 24-individual, randomized, four-period, symmetric, crossover study of human subjects, the bioavailability parameters obtained with the administration of the test composition 1 were compared with those obtained with the above-m...
Embodiment 3
[0123] Two selexib / caffeine suspensions were prepared as follows.
[0124] 1. PVP (K30) was added to water to form a PVP solution of 11.25 mg PVP / ml solution; two 12 ml portions of this PVP solution were taken and placed in separate containers (A and B).
[0125] 2. Add 180 mg of caffeine aliquots to container A and 900 mg of caffeine aliquots to container B to form caffeine suspensions A and B respectively; Sonicate.
[0126] 3. Prepare the Selixix suspension (in water) as a suspension containing 30% Selixix and PVP (K30) at a concentration of 11.25 mg PVP / ml solution; Dynomill the Selixix suspension Wet milling was performed to form a finely ground Selixix suspension.
[0127] 4. Take two 6ml portions of finely ground Selixix suspension and add them to Caffeine Suspensions A and B to form Selixix / Caffeine Suspensions A and B respectively.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight average particle size | aaaaa | aaaaa |
| Weight average particle size | aaaaa | aaaaa |
| Weight average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com