Method and apparatus for processing metals, and the metals so produced
A metal and equipment technology, applied in chemical instruments and methods, metal material coating technology, ion exchange column/bed method, etc., can solve problems such as semiconductor component failure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
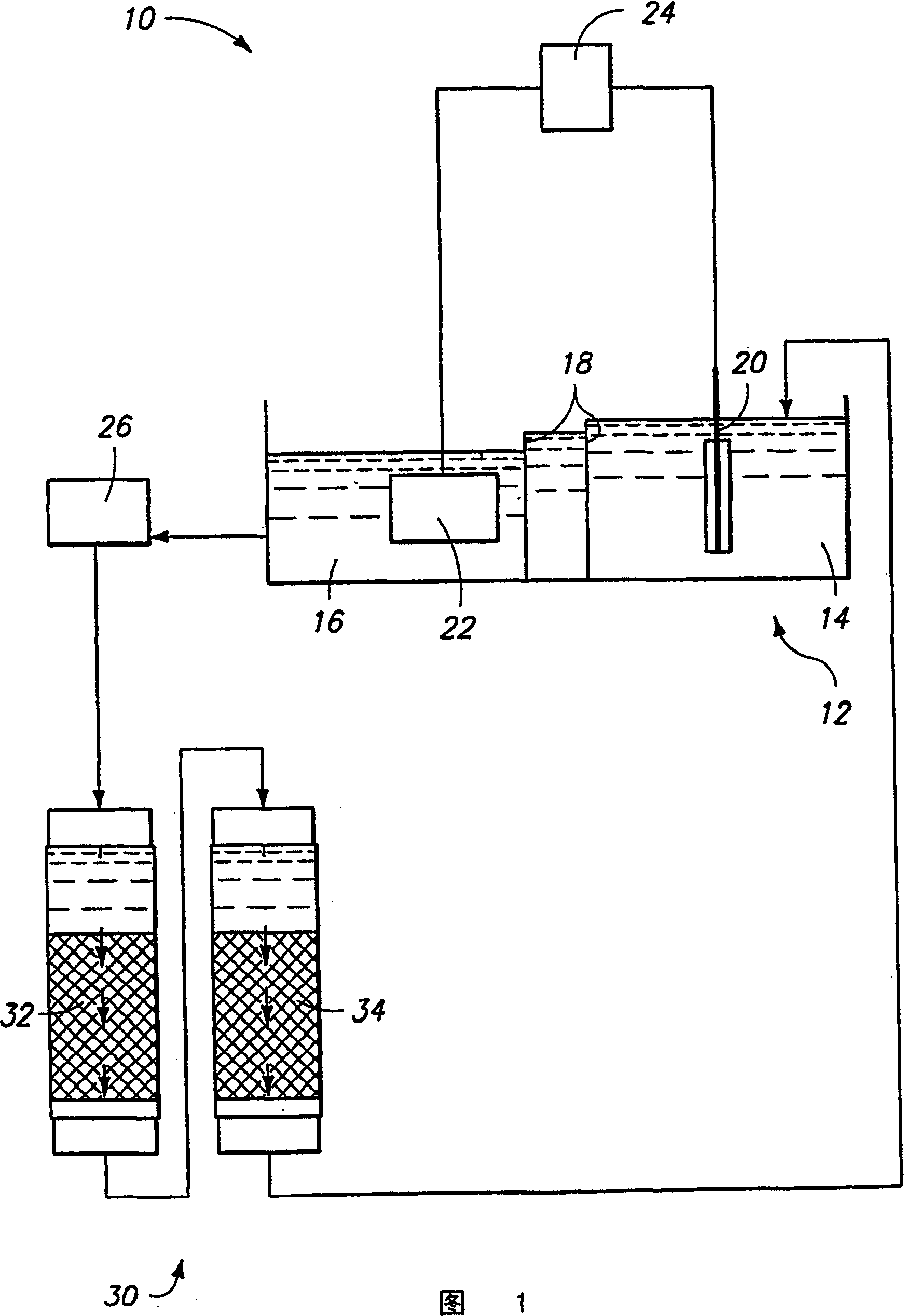
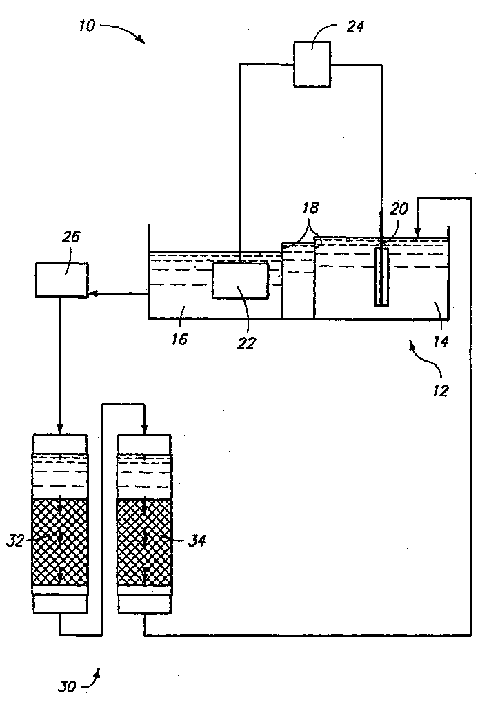
[0054] Electrolytic formation of cobalt
[0055] CoSO 4 ·7H 2A 1472 lb sample of O was dissolved in 370 gallons of water while stirring. While stirring again, the pH of the cobalt sulfate solution was adjusted to 2 by adding 2.44 gallons of 98% sulfuric acid, ACS grade. The solution was added to a separate electrolytic cell and heated to 122°F. Circulation was initiated to an ion exchange tank containing 5 cubic feet of resin with a flow rate of 0.5 GPM through the tank. The cobalt sulfate solution was analyzed and found to contain 80-90 g / L Co, 3-4 mg / L Fe, 1-2 mg / L Ni, and a pH of 2. The electrolysis was performed at a constant current of 300 A, and a voltage drop from 9 V to 5 V was observed after 216 hours of operation. Cathode is 99.95% Co plate at 18A / ft 2 work at current densities. Approximately 116 lbs of cobalt were obtained, which corresponds to a cathode current efficiency of 74%. The analysis results of the deposited layer are shown in Table 1 as "High Puri...
Embodiment 2
[0058] CoCl 2 system
[0059] Cobalt powder with a purity of 3N8 (99.98%), powder A, and 2N7 (99.7%), powder B were dissolved in HCl (35-38% by weight in water). The solution was then heated to about 80°C with stirring for about 10 hours. Add 2 L of deionized water to dissolve the solid CoCl 2 ·6H 2 O was dissolved and stirred at about 50°C for about 8 hours. More deionized water was then added to obtain a final solution volume of about 5 liters.
[0060] A plastic tube with an inner diameter of 0.953 cm and a length of 120 cm was connected to one end of the pressure reducer and used as an ion exchange column. Glass wool is used as the screen material. The tube was filled with approximately 42.6 ml of Dowex M-4195 anion exchange resin having an average size of 20-50 mesh. Prior to loading, it was conditioned by passing 2 bed volumes (BV) of HCl solution through the resin at a flow rate of approximately 15 BV / Hr. The pH of the HCl solution is the same as the pH of the d...
Embodiment 3
[0070] Fe removal
[0071] Fe is the main impurity element in cobalt. Like Ni, it affects the breakthrough flux of the cobalt sputtering target and is therefore preferably minimized. Although the resin employed in the present invention has the ability to absorb a certain amount of Fe, an additional Fe removal step is required when the Fe content in the raw cobalt is high. Fe can be removed in different ways: 1) Fe(OH) 3 precipitation; 2) solvent extraction; 3) additional selective ion exchange; and so on. In particular instances, this method has successfully converted Fe(OH) 3 The precipitation of Fe is incorporated in the cobalt refining process to deal with excess Fe impurities.
[0072] For Fe(OH) 3 For precipitation, blow air or oxygen into the impure CoSO during stirring 4 or CoCl 2 solution for a certain period of time, so that the Fe 2+ Ions are oxidized to Fe 3+ ion. Then add NaOH to CoSO 4 or CoCl 2 solution to change its pH to about 4. Due to Fe(OH) 3 L...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com