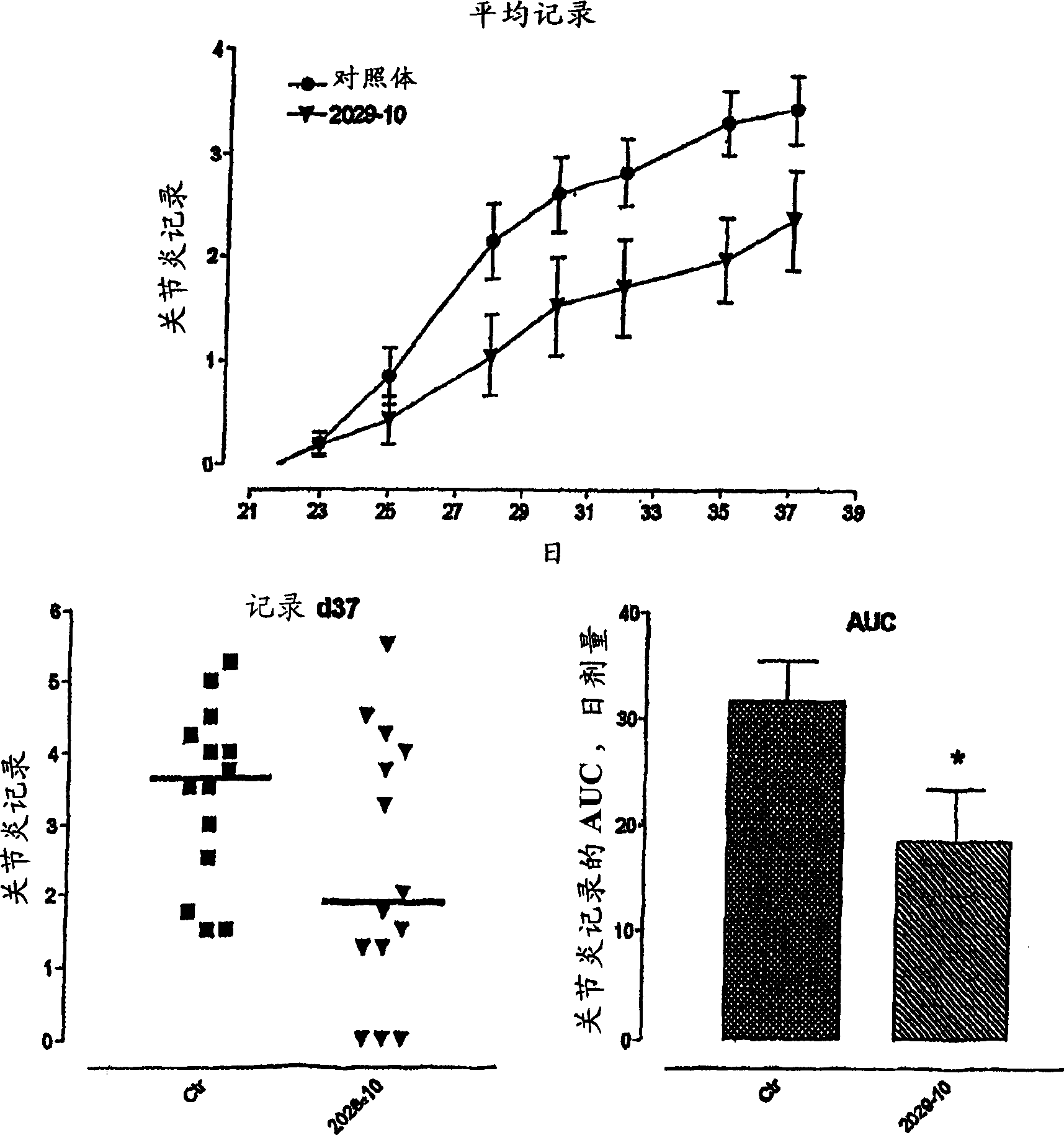
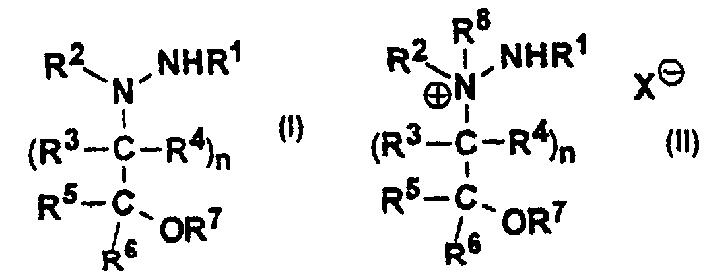Inhibitors of copper-containing amine oxidases
A technology of amine oxidase and compounds, applied in the field of enzyme inhibitors, can solve problems such as not showing activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0116] Example 1 (1R, 2S)-2-(1-methylhydrazino)-1-phenyl-1-hydrogen maleate (1)
[0117] In an ice bath with vigorous stirring, to a suspension of (1R,2S)-2-methylamino-1-phenyl-1-propanol hydrochloride (2.02g, 10mmol) in water (50ml) was added dropwise NaNO 2 (1.38g, 20mmol) in water (10ml), followed by the dropwise addition of AcOH (0.30g, 5mmol). The mixture was stirred at room temperature for 8 hours, then extracted with EtOAc (4 x 50ml). dry (Na 2 SO 4 ) through the combined organic phases, and evaporated under reduced pressure to obtain 1.86 g of oily product of N-nitroso derivatives, which were directly used in subsequent steps without further purification.
[0118] To the stirred LiAlH 4 (1R, 2S)-2-methylamino-N-nitroso-1-phenyl-1-propanol (1.86 g, 9.6 mmol) was added dropwise to a suspension of (0.73 g, 19.2 mmol) in THF (50 ml). ) in THF (20ml), the mixture was stirred and refluxed for 3 hours. with H 2 A mixture of O (1.5ml) and THF (20...
Embodiment 2
[0119] Embodiment 2 (1R * , 2S * )-2-(1-methylhydrazino)-1-phenyl-1-propanol hydrochloride (2)
[0120] Add (1R * , 2S * )-2-Methylamino-1-phenyl-1-propanol (0.86g, 5.3mmol) in ether (5ml). The resulting reaction mixture was stirred at room temperature for 30 minutes and evaporated to dryness. To the residue was added 5% hydrochloric acid (30ml), and the mixture was stirred at ambient temperature for 1 hour. with Et 2 O (2×30ml) washed the mixture with Na 2 CO 3 Basified and extracted with EtOAc (3 x 50ml). The combined EtOAc extracts were dried and evaporated to give a solid residue which was dissolved in methanol (5ml) and converted to the crystalline salt with 22% ethanolic hydrochloric acid (2ml) and diethyl ether salt. The crystals were filtered off and recrystallized from methanol / ether. 1 H-NMR (400MHz, D 2 O) δ (ppm): 1.20 (3H, d, J = 6.8Hz, CHCH 3 ), 3.09 (3H, s, NCH 3 ), 3.67 (1H, m, NCH), 5.41 (1H, br s, OCH), 7.47 (5H, m, C 5 h ...
Embodiment 3
[0121] Embodiment 3 (1R * , 2S * )-2-(1-methylhydrazino)-1-phenyl-1-propanol hydrochloride (2)
[0122] Add dropwise (1R * , 2S * )-2-(1-methylhydrazino)-N-nitroso-1-phenyl-1-propanol (1.94g, 10mmol, prepared according to Example 1 from (1R * , 2S * )-2-Methylamino-1-phenyl-1-propanol hydrochloride) in AcOH (18ml). The temperature of the reaction mixture was maintained at 20-25°C during the addition by external cooling. After the addition was complete, the mixture was stirred at 50 °C for 1 hour, then filtered with suction, and the zinc residue was washed with H 2 A mixture of O (15ml) and AcOH (5ml) was washed. The filtrate and washings were combined and concentrated in vacuo to about 10 mL. Under ice-cooling, the solution was basified with NaOH solution, and then washed with Et 2 O (4 x 50ml) extraction. The combined ether extracts were dried and evaporated to give a yellow oil which was dissolved in methanol (5ml) and converted to the crystal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com