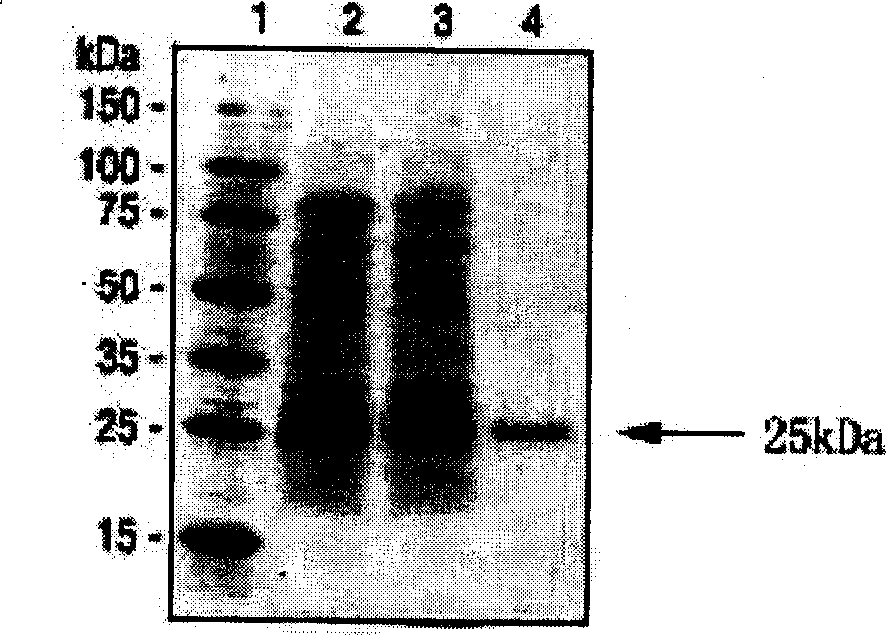
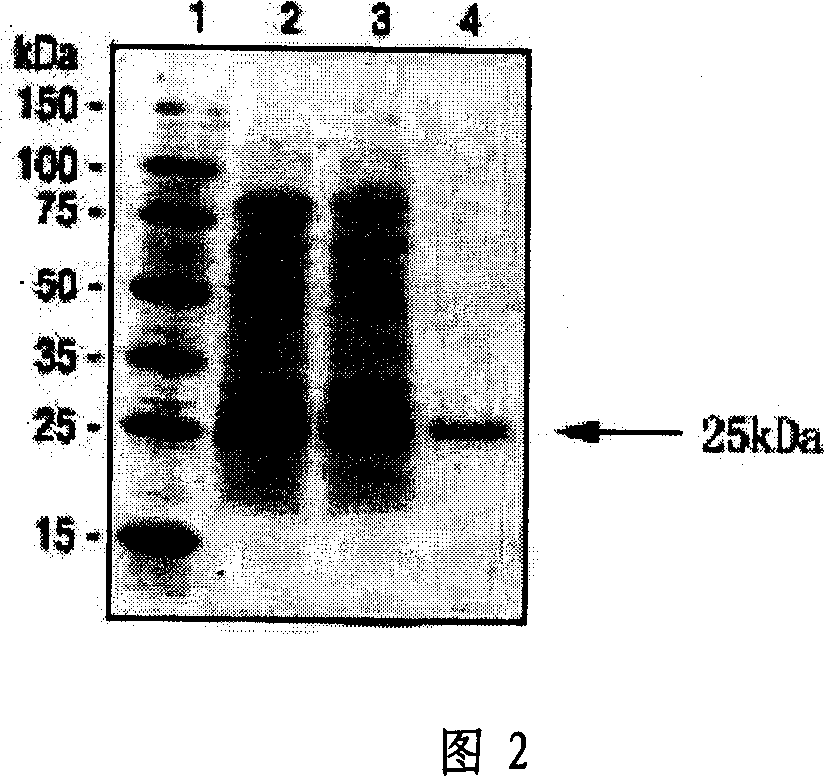
Polypeptide-human transmembrane glycopeptide 25 and polynucleotide for encoding such polypeptide
A transmembrane glycoprotein and polynucleotide technology, applied in the direction of peptide/protein composition, anti-animal/human immunoglobulin, peptide source, etc., can solve problems such as cell differentiation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0116] Example 1: Cloning of human transmembrane glycoprotein 25
[0117] Total RNA was extracted from human fetal brain by one-step method of guanidine isothiocyanate / phenol / chloroform. Poly(A) mRNA was isolated from total RNA using Quik mRNA Isolation Kit (product of Qiegene). 2ug poly(A) mRNA was reverse transcribed to form cDNA. Smart cDNA cloning kit (purchased from Clontech) was used to insert the cDNA fragment into the multiple cloning site of the pBSK(+) vector (product of Clontech Company), transform DH5α, and the bacteria formed a cDNA library. The sequences of the 5' and 3' ends of all clones were determined using Dyeterminate cycle reaction sequencing kit (product of Perkin-Elmer) and ABI 377 automatic sequencer (Perkin-Elmer). Comparing the determined cDNA sequence with the existing public DNA sequence database (Genebank), it was found that the cDNA sequence of one of the clones, 1000A03, was a new DNA. The insert cDNA fragment contained in this clone was deter...
Embodiment 2
[0118] Example 2: Homology retrieval of cDNA clones
[0119] The sequence of the human transmembrane glycoprotein 25 of the present invention and the protein sequence encoded by it, using the Blast program (Basiclocal Alignment search tool) [Altschul, SF et al.J.Mol.Biol.1990; 215:403-10], Homology searches were performed in databases such as Genbank and Swissport. The gene with the highest homology to human transmembrane glycoprotein 25 of the present invention is a known mouse shc protein, and the accession number of the encoded protein in Genbank is U15784. The results of protein homology are shown in Figure 1. The two are highly homologous, with an identity of 52% and a similarity of 64%.
Embodiment 3
[0120] Example 3: Cloning of the gene encoding human transmembrane glycoprotein 25 by RT-PCR
[0121] The total RNA of fetal brain cells was used as a template, and oligo-dT was used as a primer to carry out reverse transcription reaction to synthesize cDNA. After purification with a Qiagene kit, PCR amplification was performed with the following primers:
[0122] Primer1: 5'-AACACATGGCTTACTGCCCCATAC-3'(3)
[0123] Primer2: 5'-CGAATTCAATGTCATATTTATTTT-3'(4)
[0124] Primer1 is the forward sequence starting from the 1bp at the 5' end of 1;
[0125] Primer2 is the 3' reverse sequence in 1.
[0126] The conditions of the amplification reaction: 50mmol / L KCl, 10mmol / L Tris-Cl, (pH8.5), 1.5mmol / L MgCl in a reaction volume of 50μl 2 , 200 μmol / L dNTP, 10 pmol primer, 1 U of Taq DNA polymerase (product of Clontech Company). On a PE9600 DNA thermal cycler (Perkin-Elmer), the reaction was performed for 25 cycles under the following conditions: 94°C for 30 sec; 55°C for 30 sec; 72°C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com