Specific monoclonal antibody against epitope of clygoprotein coding inactivated feline immunodeficiency virus
A monoclonal antibody and glycoprotein technology, applied in the direction of immunoglobulin, antiviral immunoglobulin, viral antigen components, etc., can solve the problems of inability to recognize and inactivate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Preparation of Monoclonal Antibodies Specific for Inactivated FIV-Encoded Glycoprotein Epitopes
[0024] cells and viruses
[0025] FIV-Shizuoka (FIV-Shiz, D FIV subtype) is propagated in a persistently infected lymphoid cell line obtained from FIV-Shizuoka and Fet-J (ATCC deposit number CRL 11967), which is an independent The cell line for IL-2, called Shiz. A cell line persistently infected with FIV-Shizuoka is also deposited with the ATCC under accession number CRL 11976. To generate an antigen stock solution, the virus solution was inactivated with formalin and concentrated by ultrafiltration.
[0026] Antigen stocks can also be prepared from various other FIV strains and subtypes in a similar manner, such as wild isolates, FIV strain NCSU 1 (ATCC deposit number VR-2333), FIV strain UC24818 (ATCC deposit number VR-2619), FIV- Petaluma (subtype A, TCC deposit number VR-2186), FIV-Dixon (subtype A), FIV-UK8 (subtype A), FIV-Bangston (subtype B), FIV-Amori-1 (subtyp...
Embodiment 2
[0030] Application of Monoclonal Antibody mAb 1D9 as Detection Antibody in Enzyme-Linked Immunosorbent Assay
[0031] In this evaluation, Galanthus Nivalis lectin (GNA) was used to capture glycoproteins. This GNA ELISA combines high selectivity for GNA binding with its broad reactivity with HIV-1, HIV-2, SIV and FIV glycoproteins. First, a 96 microwell plate was coated with 10 pg / mL GNA (dissolved in 50 mM carbonate) at pH 9.6 at 37° C. for 1 hour. After the wells were blocked with PBS-10% FBS at 37°C for 2 hours, samples treated with 1% Empigen BB (Calbiochem) at 37°C for 1 hour were added and incubated at 37°C for 2 hours. Unbound antigen was removed by washing 3 times with PBS containing 0.1% Tween 20. The monoclonal antibody mAb 1D9 of Example 1 diluted 1:8000 was added to each well, and the plate was incubated at 37°C for 1 hour. After washing, a 1:1000 dilution of peroxidase-labeled goat anti-mouse IgG Kirkegaard & Perry Laboratories (KPL) was added and the plate was ...
Embodiment 3
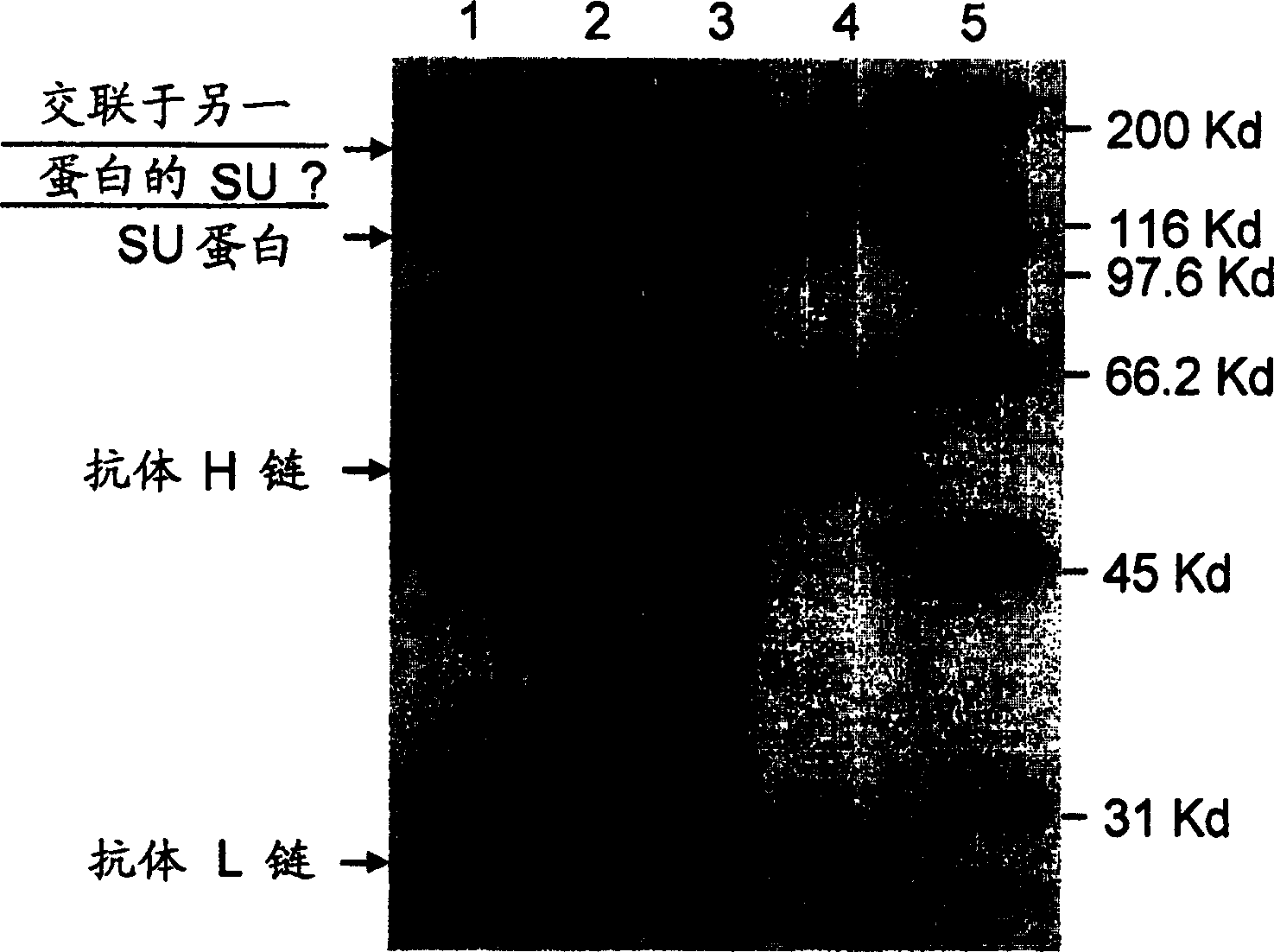
[0033] Use of monoclonal antibody mAb 1D9 as a detection antibody in immunoprecipitation assays
[0034] In this evaluation, a virus stock solution enriched with formalin-inactivated FTV-Petaluma virus (less than 5% FIV protein in 0.38 mg total protein) and 5 mg Thio-NHS-LC-Bio Pierce was incubated on ice for 1 hour. After removal of unincorporated biotin reagent by dialysis, virus-containing samples were extracted with 1% Triton X-100 in 12 mL of PBS for 1 hour and centrifuged at 100,000 g for 2 hours. The supernatant was recovered and used for immunoprecipitation. Immunoprecipitation was achieved by incubating 600 μl of extracts with 80 μl of mAb 1D9 or mAbH5332 for 1 hour at 4°C. mAB H5332 (specific for Borrelia OspA protein) was used as an irrelevant antibody control. Immune complexes were collected on immobilized protein G (Pierce), washed 4 times with cold PBS-1% NP-40, resuspended in Laemmli buffer, and subjected to SDS-PAGE and Western blotting. Blots were blocked ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com