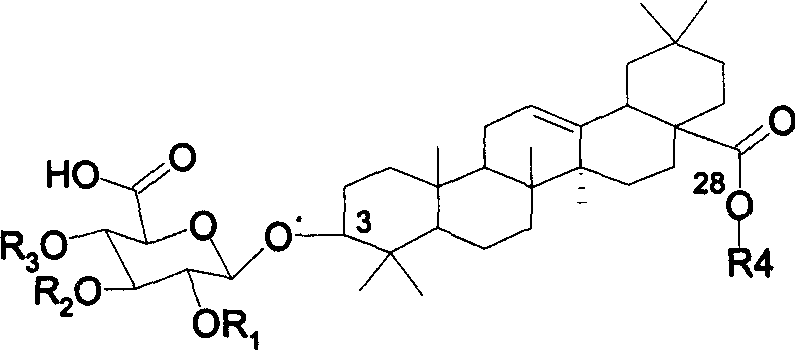
Chemosynthesis of glucuronide oleanane type double sugar chains triterpenoid saponin
A technology of aldoside oleanane and triterpene saponins, which is applied in the field of synthesis of oleanane-type pentacyclic triterpene saponins, and can solve problems such as limited synthesis reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
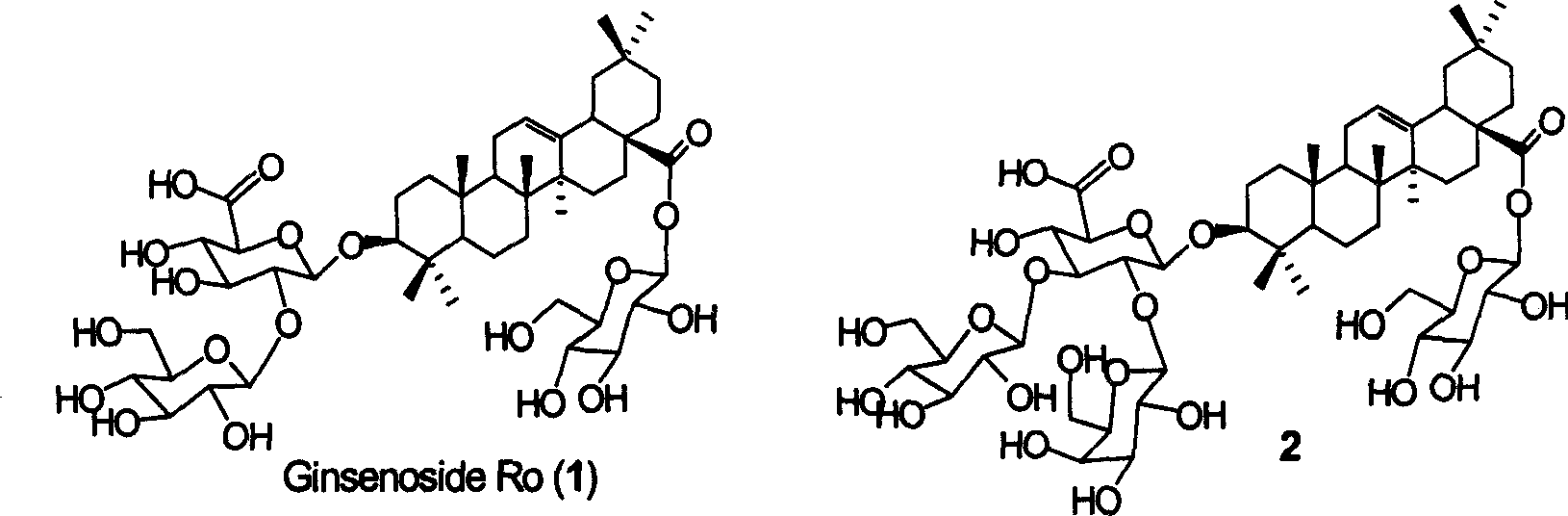
[0027] Synthesis of Ginsenoside Ro(1)
[0028] The synthetic route is shown in the figure below:
[0029]
[0030] Reagents and conditions: (a) K 2 CO 3 , Bu 4 NBr, CH 2 Cl 2 -H 2 O, reflux, 90%. (b) TBSOTf (0.1 equiv), CH 2 Cl 2 , 4 MS, rt, 91%.(c)Bu 3 P, THF-H 2 O, 75%. (d) TBSOTf (0.4 equiv), CH 2 Cl 2 , 4 Å MS, rt, 79%. (e) AcCl, MeOH-CH 2 Cl 2 , 0°C-rt, 89%. (f) TEMPO, Ca(ClO) 2 , KBr, Bu 4 NBr, CHCl 3 -H 2 O, 0°C, 89%. (g) NaOMe, MeOH-CH 2 Cl 2 , 72%.
[0031] Specific experiments and data:
[0032] (1) 2,3,4,6-Tetra-O-benzoyl-β-D-glucopyranosyl oleanolic ester (4): oleanolic acid (195mg, 0.43mmol) and 2,3,4,6-tetra- O-Benzoyl-α-D-glucobromoside (377 mg, 1.3 equiv) was dissolved in CH 2 Cl 2 (5.0mL) and water (5.0.mL) two-phase system, and add K 2 CO 3 (151mg, 2.5 equivalents) and Bu 4 NBr (56 mg, 0.4 eq) was then heated to reflux until TLC showed the reaction was complete. The reaction system uses CH 2 Cl 2 Diluted, then...
Embodiment 2
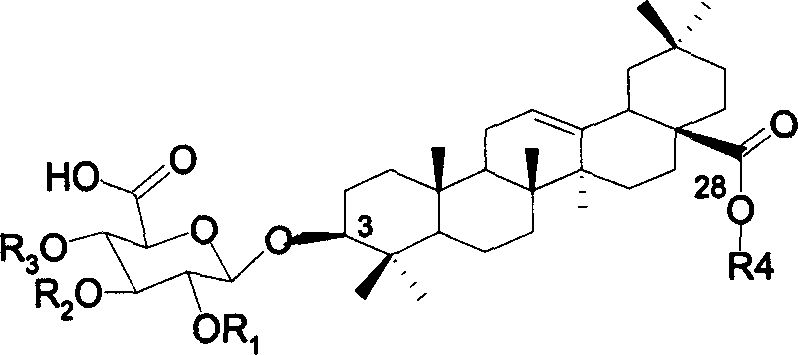
[0040] Synthesis of GOTCAB Saponin 2
[0041] The synthetic route is shown in the figure below:
[0042]
[0043] Reagents and conditions: (a) TBSOTf (0.1 equiv), CH 2 Cl 2 , 4 MS, rt, 89%.(b) Bu 3 P, THF-H 2 O, 90%. (c) TBSOTf (0.4 equiv), CH 2 Cl 2 , 4 Å MS, rt, 90%. (d) AcCl, MeOH-CH 2 Cl 2 , 0°C-rt, 88%. (f) TEMPO, Ca(ClO) 2 , KBr, Bu 4 NBr, CHCl 3 -H 2 O, 0°C, 85%. (g) NaOMe, MeOH-CH 2 Cl 2 , 78%.
[0044] Specific experiments and data:
[0045] (1) 2,3,4,6-Tetra-O-benzoyl-β-D-glucopyranoside-3-O-[2,3,4,6-tetra-O-benzoyl-β-D-glucopyranosyl-(1 → 3)-4,6-di-O-acetyl-2-O-(2-azidomethyl)benzoyl-β-D-glucopyranosyl]oleanolic ester (13): the donor 12 (1.0g, 1.1 equivalents) and the acceptor Enzyme 4 (806 mg, 0.779 mmol) was dissolved in anhydrous CH 2 Cl 2 and add 4 Å molecular sieves. Under the protection of argon, TBSOTf (0.1 equivalent) was added dropwise to the system. After stirring at room temperature for 3 hours, the react...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com