Process for isolation of monophenolic-bisaryl triazines
A technology of aryl and aroyl, applied in the field of separation of monohydric phenol-diaryl triazine compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
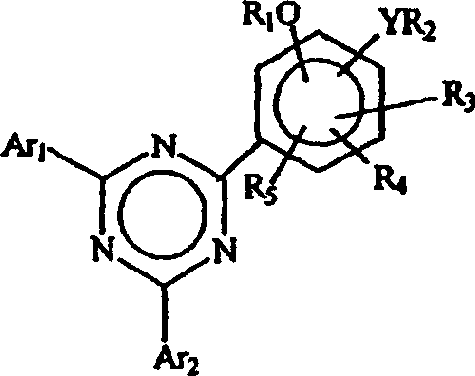
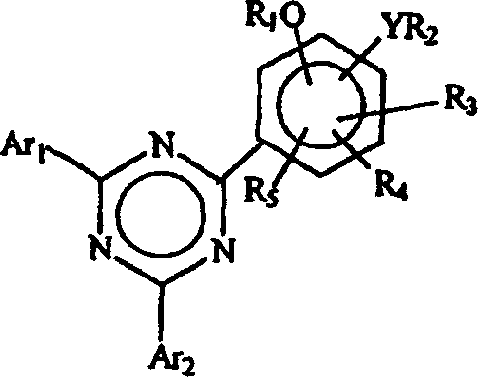
[0094] Example 1: Treatment with 5% aqueous sodium carbonate to isolate 2-(2,4-dihydroxyphenyl)- 4,6-bis(2,4-dimethylphenyl)-1,3,5-triazine
[0095] a. Preparation of 2-(2,4-dihydroxyphenyl)-4,6-bis(2,4-dimethylphenyl)-1,3,5-triazine (according to WO 00 / 29392)
[0096] A reaction flask equipped with a reflux condenser, nitrogen inlet and mechanical stirrer was charged with 50 g cyanuric chloride, 191 ml o-dichlorobenzene (ODCB) and 108.5 gm aluminum chloride. The mixture was placed in an ice bath, cooled to 5°C, and then 6.5 gm of concentrated hydrochloric acid was added over 20 minutes. The mixture was warmed to room temperature and stirred for 2 hours. It was cooled again to 5°C and then 51.8 gm of m-xylene was added slowly over 4 hours while allowing the temperature to rise to 21°C. The mixture was stirred at room temperature for another 16 hours. The reaction mixture was heated to approximately 69°C and 32.8 gm m-xylene was added over 30 minutes. The mixture wa...
Embodiment 2
[0100] Example 2: Isolation of 2-(2,4-dihydroxyphenyl)-4,6-bis by treatment with 3% aqueous sodium carbonate (2,4-Dimethylphenyl)-1,3,5-triazine
[0101] Add 112.5 ml of 3% aqueous sodium carbonate solution and 50 gm of the crude polyresorcinol-triazine impurity prepared in Example 1a to a reaction flask equipped with a reflux condenser, a Dean-Stark apparatus, a nitrogen inlet, and a mechanical stirrer. 2-(2,4-Dihydroxyphenyl)-4,6-bis(2,4-dimethylphenyl)-1,3,5-triazine. The resulting mixture was heated to reflux and held at reflux for 2 hours and the residual ODCB was collected as an azeotrope in a Dean-Stark apparatus. Heating was stopped, the mixture was filtered at about 80°C, and the filter cake was washed with 112.5 ml of 3% aqueous sodium carbonate and 300 ml of 50°C water. A residue of 47 g thus obtained was analyzed by HPLC as 2-(2,4-dihydroxyphenyl)-4,6-bis(2,4-dimethylphenyl)- 1,3,5-Triazine.
[0102] a. Separation of polyresorcinol-triazines
[0103] The ...
Embodiment 3
[0104] Example 3: Treatment with aqueous sodium hydroxide solution isolated 2-(2,4-dihydroxyphenyl)-4,6-bis (2,4-Dimethylphenyl)-1,3,5-triazine
[0105]Into a reaction flask equipped with a reflux condenser, a Dean-Stark apparatus, a nitrogen inlet, and a mechanical stirrer was added 50 gm of the crude 2-(2,4-dihydroxy phenyl)-4,6-bis(2,4-dimethylphenyl)-1,3,5-triazine, followed by the addition of 175 ml of 0.25% aqueous sodium hydroxide solution. The reaction mixture was heated to reflux and the residual ODCB was collected as an azeotrope in a Dean-Stark apparatus. An additional 175 ml of 0.25% aqueous sodium hydroxide solution was added to maintain the pH at about 10, and reflux was continued for 1 hour. Heating was stopped, the mixture was filtered, and the filter cake was washed successively with 30 ml of 0.25% aqueous sodium hydroxide solution and 500 ml of water. Analysis of the product (47 gm) by HPLC revealed no detectable residues in 2-(2,4-dihydroxyphenyl)-4,6-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com