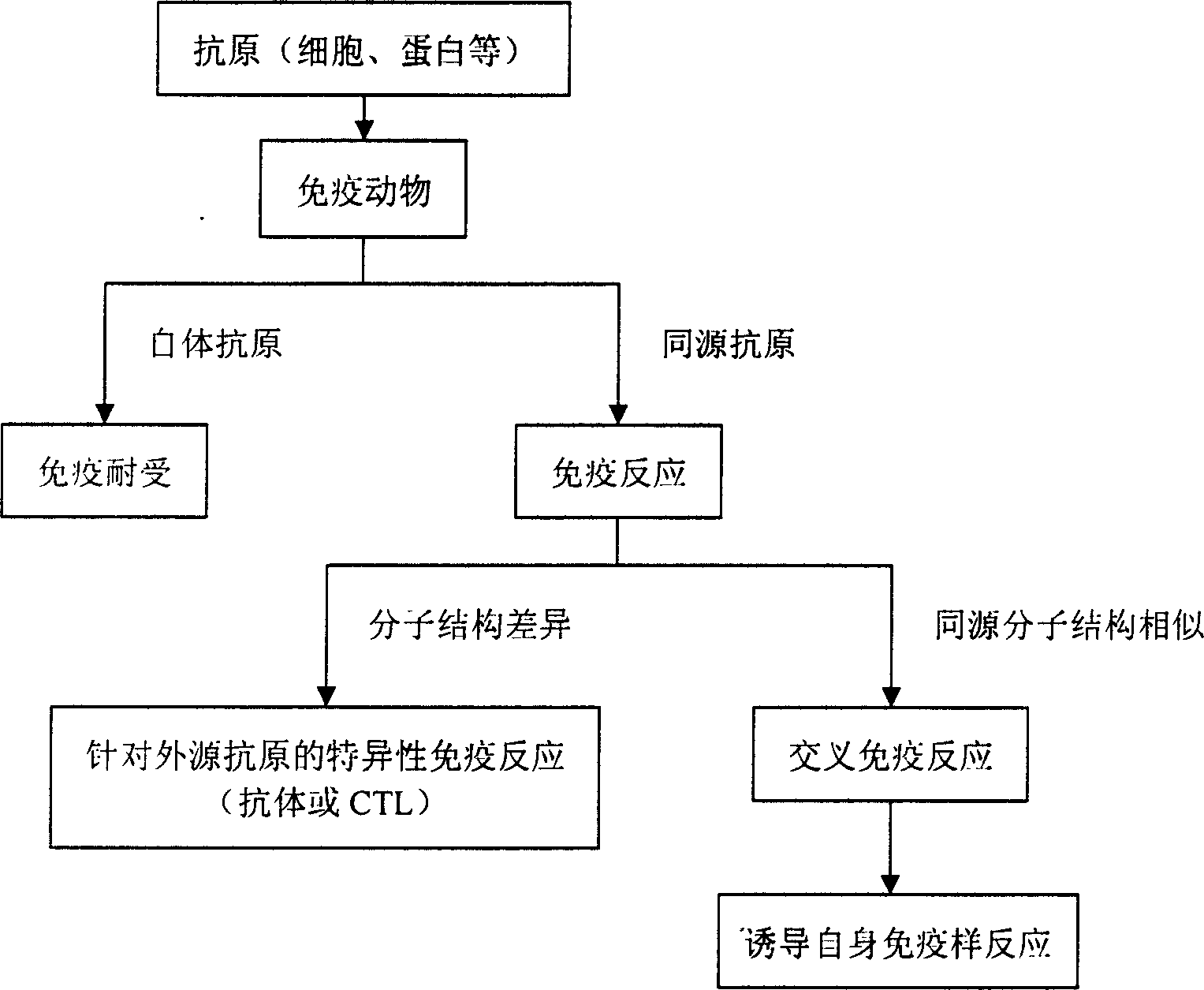
Vaccine preparing process and antitumor vaccine
A technology of vaccines and tumor cells, applied in the biological field, can solve problems such as pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
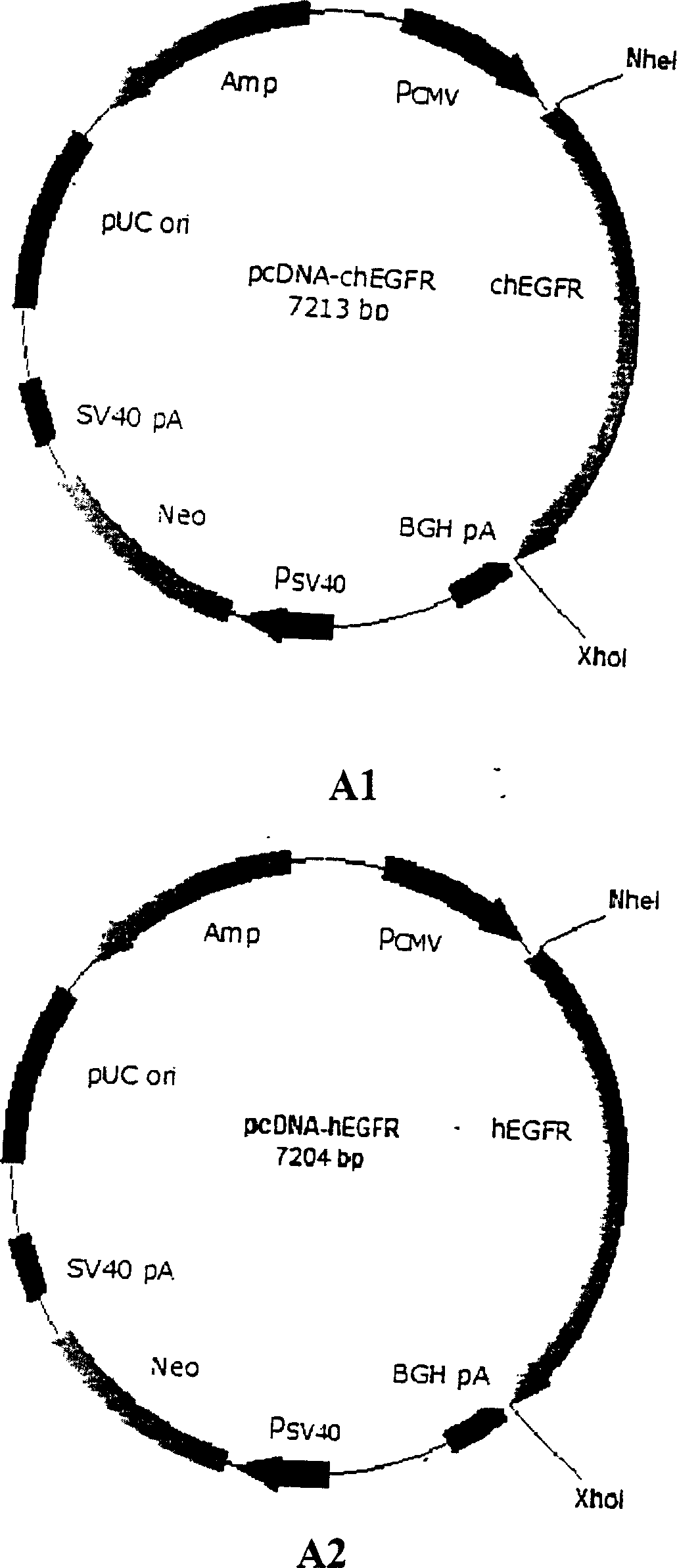
[0155] Example 1 EGFR recombinant DNA vaccine
[0156] Design PCR primers (human primers are: 5) according to the cDNA sequences of human, mouse, chicken, and EGFR molecules collected in various public databases such as GenBank (corresponding to SEQ ID NO 1-5, 7-9, 19) 'GACCATGGAGGAAAAGAAAGTTTGC 3', 5'ACGAATTCTTAGGACGGGATCTTAGGCCCA3'; the primers for mice are: 5'GACCATGGAGGAAAAGAAAGTCTGC 3', 5'ACGAATTCTTAATAGATGGTATCTTTGGC 3'; the primers for chicken are: 5'GACCATGGAGAGTTAGAGGATTAG, GTTTAGGAGTTAG, GTTTAG, human lung cancer, respectively The total RNA of cell line A431, mouse lung cancer cell line LL2 and chicken embryo were used as templates for RT-PCR amplification, and the amplified EGFR fragments (all 1.9 kb) were collected and purified by electrophoresis, and then the PCR products were subcloned. After confirming the PCR subclones and sequencing, they were digested with NcoI and EcoRI, and the 1.9 kb fragment was collected and purified, and inserted into the pORF-MCS (InvivoGe...
Embodiment 2
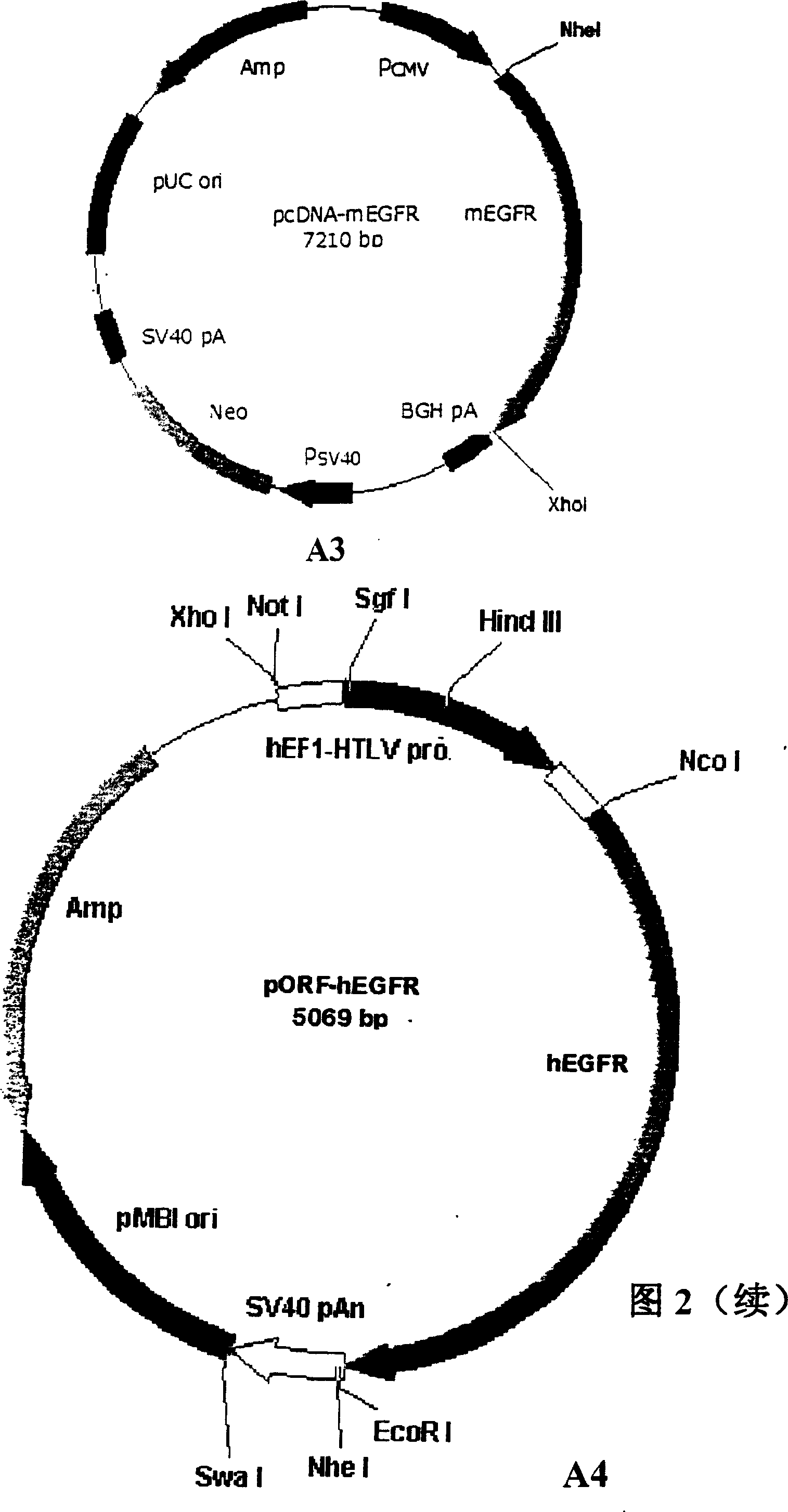
[0161] Example 2 EGFR recombinant protein vaccine (E. coli expression)
[0162] As mentioned above, the PCR primers (human, mouse, chicken, and EGFR molecule cDNA sequences (corresponding to SEQ ID NO 1-5, 7-9, 19) collected in GenBank and other public databases, respectively) are used to design PCR primers (human The primers are: 5'GACCATGGAGGAAAAGAAAGTTTGC 3', 5'ACAGATCTAGGACGGGATCTTAGGCCCA 3'; the primers for mice are: 5'GACCATGGAGGAAAAGAAAGTCTGC 3', 5'ACAGATCTATAGATGGTATCTTTGGC 3'; the primers for chicken are: GTCAGACCTAGTAGGTTAGTTACAGAGTAGTGTTAG '), using pORF-hEGFR, pORF-mEGFR and pORF-chEGFR as templates for PCR amplification, electrophoresis to collect and purify the amplified EGFR fragments (all 1.9 kb), and then perform PCR product subcloning. After the PCR subclones were confirmed by sequencing, they were digested with NcoI and BglII, and the 1.9 kb fragment was collected and purified, and inserted into the pQE60 (QIAGEN company) vector digested with NcoI and BglII, and...
Embodiment 3
[0166] Example 3 EGFR recombinant protein vaccine (Yeast Pichia pastoris expression)
[0167]As mentioned above, the PCR primers (human, mouse, chicken, and EGFR molecule cDNA sequences (corresponding to SEQ ID NO 1-5, 7-9, 19) collected in GenBank and other public databases, respectively) are used to design PCR primers (human The primers are: 5'ATACTCGAGAAAAGAGAGCTGGAGGAAAAGAAAG 3', 5'GCTCTAGAATGGCACAGGTGGCACA 3'; the primers for mouse are: 5'ATGCTCGAGAAAA GAGAGTTGGAGGAAAAGAAAGTC 3', 5'AAGCGGCCGCCATAGATGGTATCTTTG 3'; the primers for chicken are: AAAAGAGTTTAGGATTAGTAGAAGTTAGTAGGTAGGTAGGAGTTAG 3'5AGAGAGTTTAGGATTAGTAGATGAGTTAGTAGAGTTAGTAGAGTTAGTAGAGTTAGTAAGTAG '), using pORF-hEGFR, pORF-mEGFR and pORF-chEGFR as templates for PCR amplification, electrophoresis to collect and purify the amplified EGFR fragments (all 1.9 kb), and then perform PCR product subcloning. After the PCR subclones were confirmed by sequencing, they were digested with XhoI and XbaI (for mouse clones as XhoI and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com