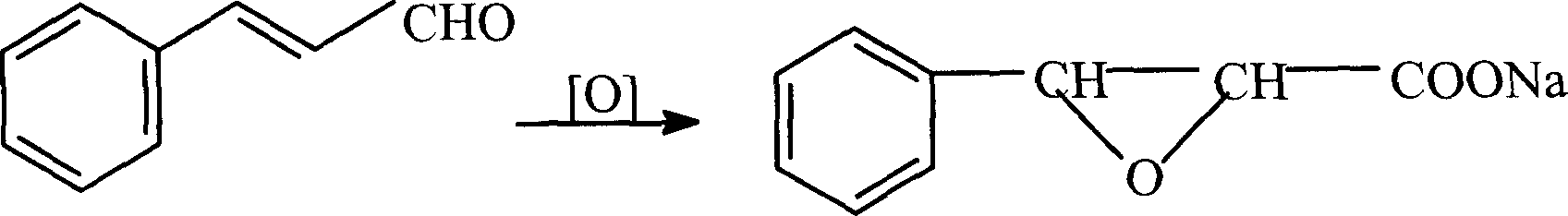
Synthesis of 3-phenyl-2,3-epoxy sodium epihydrate
A technology of sodium glycidate and a synthesis method, applied in the direction of organic chemistry and the like, can solve the problems such as easy occurrence of side reactions and reduced reaction yield, and achieve the effects of increasing reaction yield and reducing polymerization reaction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0008] Further illustrate the implementation of the present invention below:
[0009] The natural cinnamon oil is separated by a high-efficiency rectification tower to obtain natural cinnamaldehyde with a content greater than 99%, which meets the requirements of flavoring grade, and then oxidizes natural cinnamaldehyde with an oxidant under freezing and catalytic conditions to obtain 3-phenyl-2, Sodium 3-epoxypropionate.
[0010] Add 318 grams (3mol) of anhydrous sodium carbonate, 100ml water, 400ml acetic acid, 264 grams (2mol) of 99% natural cinnamaldehyde in the 2000ml there-necked flask that stirrer, balanced type dropping funnel, interior thermometer are housed, open stirring , with CaCl 2 Cool the reaction solution with brine to reduce the reaction solution to -15°C, add 760 grams of 20% peracetic acid dropwise, the internal temperature does not exceed -5°C, the dropwise addition is completed in about 5 hours, and then keep at -5°C for 5 hours. Stop stirring, filter to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com