A kind of method for preparing isobornyl acetate by esterification of camphene
A technology of isobornyl acetate and camphenyl acetate, applied in the field of chemical technology, can solve problems such as increased production cost, cumbersome process operation, low product yield, etc., and achieves the effect of improving service life and reducing polymerization reaction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
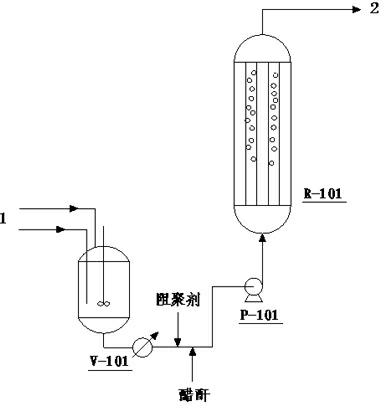
[0040] Using industrial camphene and industrial acetic acid as raw materials, put them into the raw material mixing tank V-101 at a molar ratio of 1:1.1, mix them evenly with stirring, and preheat them to 40°C with steam. The moisture content in the reactor feed was controlled to less than 50 ppm by adding acetic anhydride, and 200 ppm of hydroquinone was added. Suspended bed reactor R-101 is used, and the reactor is equipped with CT800 with an ion exchange capacity of 5.2mmol / g accounting for 80% of the reactor volume +Cation exchange resin, the resin is pre-soaked with acetic acid and acetic anhydride, and the fully mixed raw materials in V-101 are pumped into the reactor R-101 with the raw material pump P-101 at a speed of 0.2m / min, so that the catalyst is evenly suspended in the In the reactor, the reaction temperature is set at 40°C, and the feed space velocity of the reactants is 3.780 h -1 , the reaction pressure is normal pressure. The conversion rate of camphene is ...
Embodiment 2
[0042] Using industrial camphene and industrial acetic acid as raw materials, put them into the raw material mixing tank V-101 at a molar ratio of 1:2.0, mix them evenly with stirring, and preheat them to 40°C with steam. The moisture content in the reactor feed was controlled to be less than 50 ppm by adding acetic anhydride, and 50 ppm of hydroquinone was added. Suspended bed reactor R-101 is used, and the reactor is equipped with CT800 with an ion exchange capacity of 5.2mmol / g accounting for 70% of the reactor volume + Cation exchange resin, the resin is pre-soaked with acetic acid and acetic anhydride, and the raw materials that are completely mixed in V-101 are pumped into the reactor R-101 with the raw material pump P-101 at a speed of 0.1m / min, so that the catalyst is evenly suspended in the In the reactor, the reaction temperature is controlled at 20°C, and the space velocity of the reactants is 0.10 h -1 , the reaction pressure is 1.0MPa. The conversion rate of ca...
Embodiment 3
[0044] Using industrial camphene and industrial acetic acid as raw materials, put them into the raw material mixing tank V-101 at a molar ratio of 1:1.5, mix them evenly with stirring, and preheat them to 30°C with steam. By adding acetic anhydride, the moisture content in the reactor feed is controlled to be less than 50 ppm, and 100 ppm of p-benzoquinone is added. Suspension bed reactor R-101 is used, and the reactor is equipped with an ion exchange capacity of 4.8mmol / g NKC-9 catalyst accounting for 60% of the reactor volume. The resin is soaked in acetic acid and acetic anhydride in advance, and the V-101 is completely mixed. The final raw material is pumped into the reactor R-101 with the raw material pump P-101, the speed is 0.5m / min, so that the catalyst is evenly suspended in the reactor, the reaction temperature is controlled at 100°C, and the space velocity of the reactant is 6.0h -1 , the reaction pressure is normal pressure. The conversion rate of camphene is 71.2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com