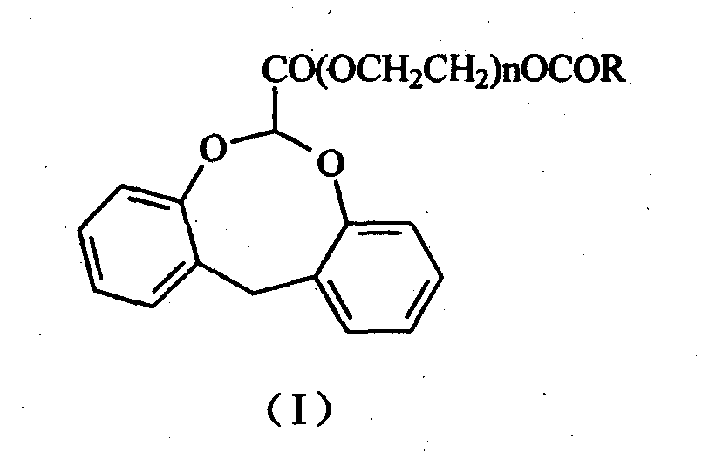
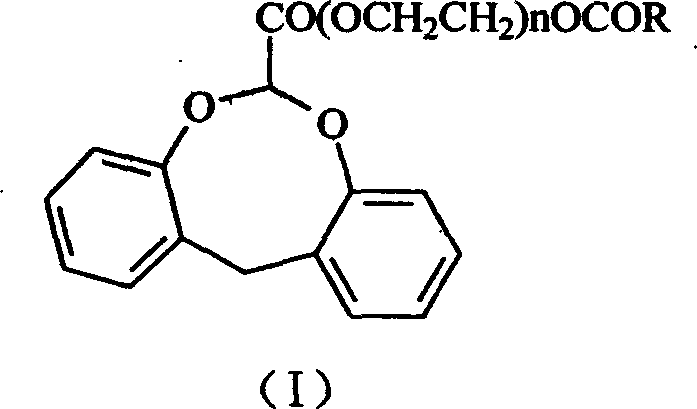
Dibenzocyclooctane carboxylic ether compounds with herbicidal activity
A technology of ester compounds and cyclooctane, which is applied in the directions of organic chemistry, animal repellent, plant growth regulator, etc., can solve the problems of unpublished bicarboxy ester compounds and so on.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example
[0124] Synthesis of Compound 9 in Table 1:
[0125]
[0126] Drop into II (7.5 grams, 0.0335mol), dichloromethane (70ml), oxalyl chloride (6.4 grams, 0.0503mol) in the reaction bottle of 150ml, absorb tail gas with lye, react at room temperature for 30 minutes, add 2 drops of DMF, and A large amount of gas was released, and the reaction was stopped after 2 hours. The reaction liquid was concentrated under reduced pressure to obtain 5.6 g of oily substance II-1.
[0127]
[0128] Add II-1 (1.0 grams, 0.00365mol) and 10ml of dichloromethane in a 50ml reaction flask, add ethylene glycol (2.2 grams, 0.0365mol) after stirring, triethylamine (0.44 grams, 0.00365mol), room temperature React for 2 hours. Pour the reaction solution into a 250ml separatory funnel, add 100ml ethyl acetate, 50ml water, separate layers, wash the ethyl acetate layer with 50ml saturated aqueous sodium bicarbonate solution, 50ml saturated aqueous sodium chloride solution, and dry over anhydrous magnesi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com