Methods of sterilizing biological materials containing non-aqueous solvents
A non-aqueous solvent and biomaterial technology, which can be used in medical preparations containing active ingredients, sanitary equipment for toilets, water supply devices, etc., and can solve problems such as loss of vitality and activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
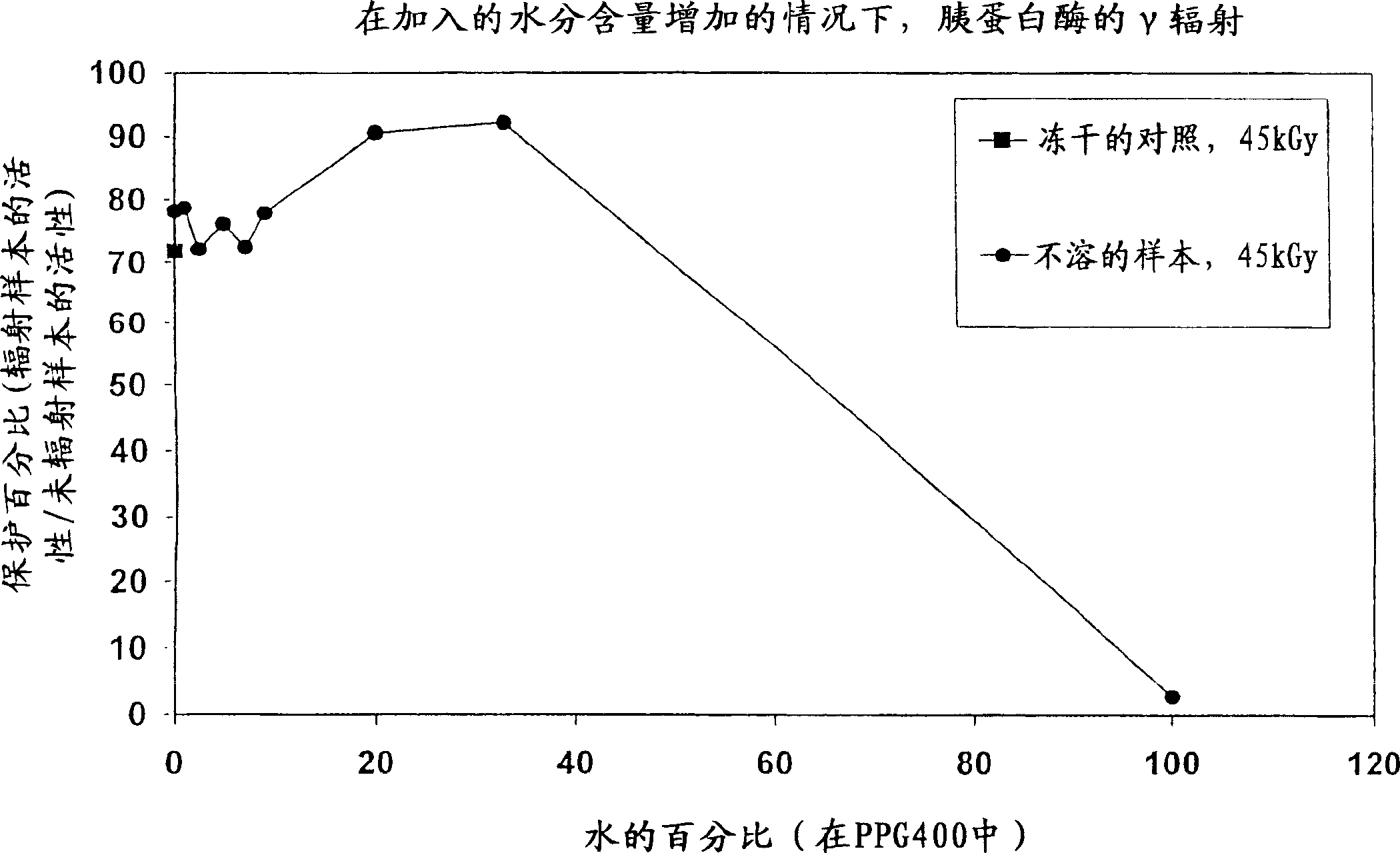
[0055] A first preferred embodiment of the present invention relates to a method for sterilizing radiation-sensitive biological material containing non-aqueous solvents, the method comprising: using radiation to effectively sterilize the material and protect the material from radiation damage The time at which the radiation rate will radiate the biological material to effectively sterilize the material.
[0056] Another preferred embodiment of the present invention relates to a method for sterilizing radiation-sensitive biological material containing non-aqueous solvents, said method comprising: (i) adding at least one stabilizer to effectively protect said biological material from the amount of radiation damage added to the biological material; and (ii) irradiating said biological material with radiation at an effective radiation rate for a time effective to sterilize the material.
[0057] Another preferred embodiment of the present invention relates to a method for steriliz...
Embodiment 1
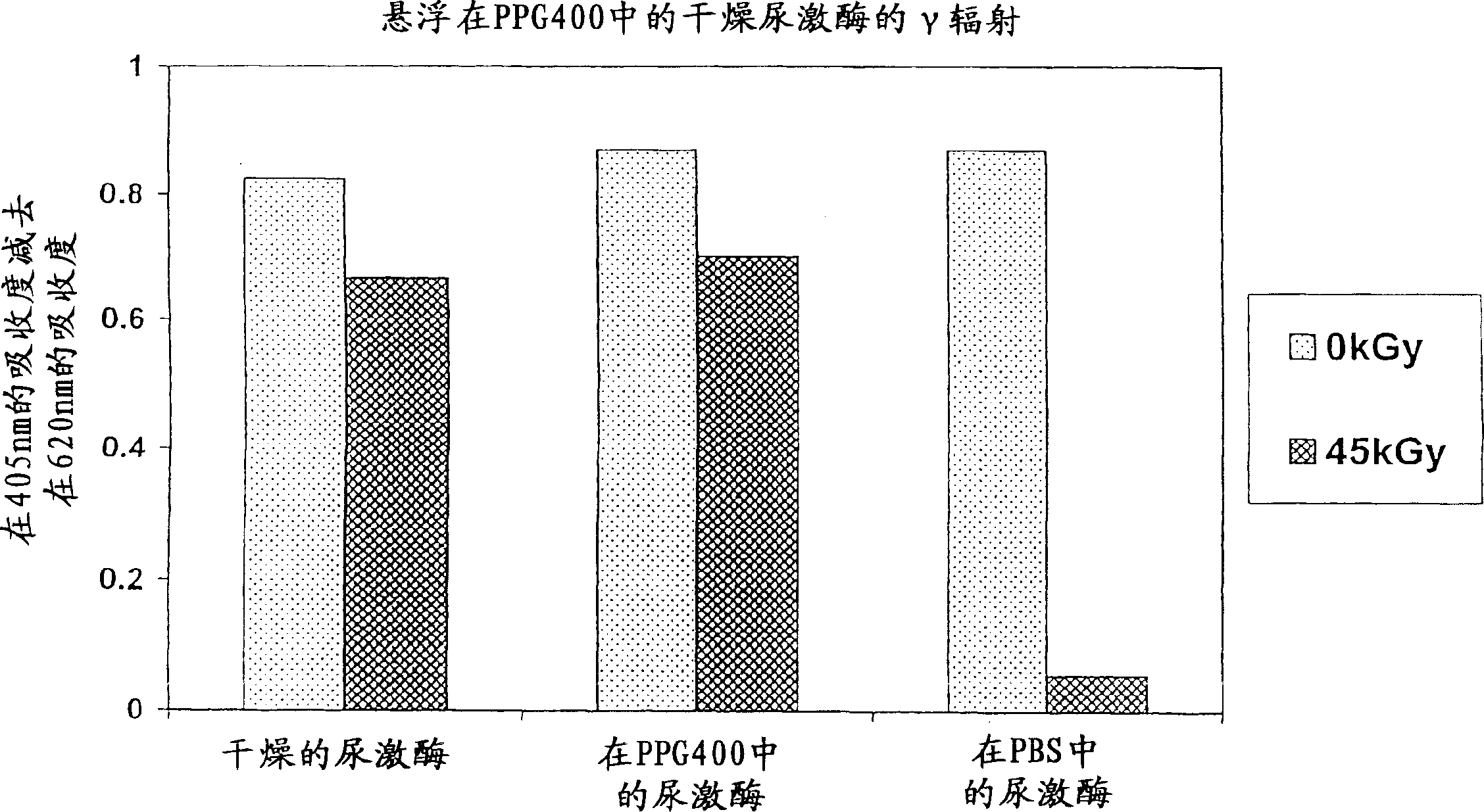
[0101] In this experiment, the effect of gamma irradiation on dried urokinase suspended in polypropylene glycol (PPG) 400 or phosphate buffered saline (PBS) was determined.
[0102] method
[0103]
Tube
sample
dry urokinase
Weight (mg)
Body of PPG400
Product (μl)
Volume of PBS
(μl)
1
Dried urokinase alone
3.2
0
0
2
Urokinase suspended in PPG400
3.16
126
0
3
Urokinase suspended in PBS
3.08
0
123
4
Dried urokinase alone
3.38
0
0
5
Urokinase suspended in PPG400
3.3
132
0
6
Urokinase suspended in PBS
3.52
0
141
[0104] After irradiation, samples were centrifuged at 14k RPM for 5 minutes at room temperature. The PPG400 solvent was removed from tubes 2 and 5, and 120 μl of PBS was added to both tubes. 128 μl and 135 μl of PBS (4...
Embodiment 2
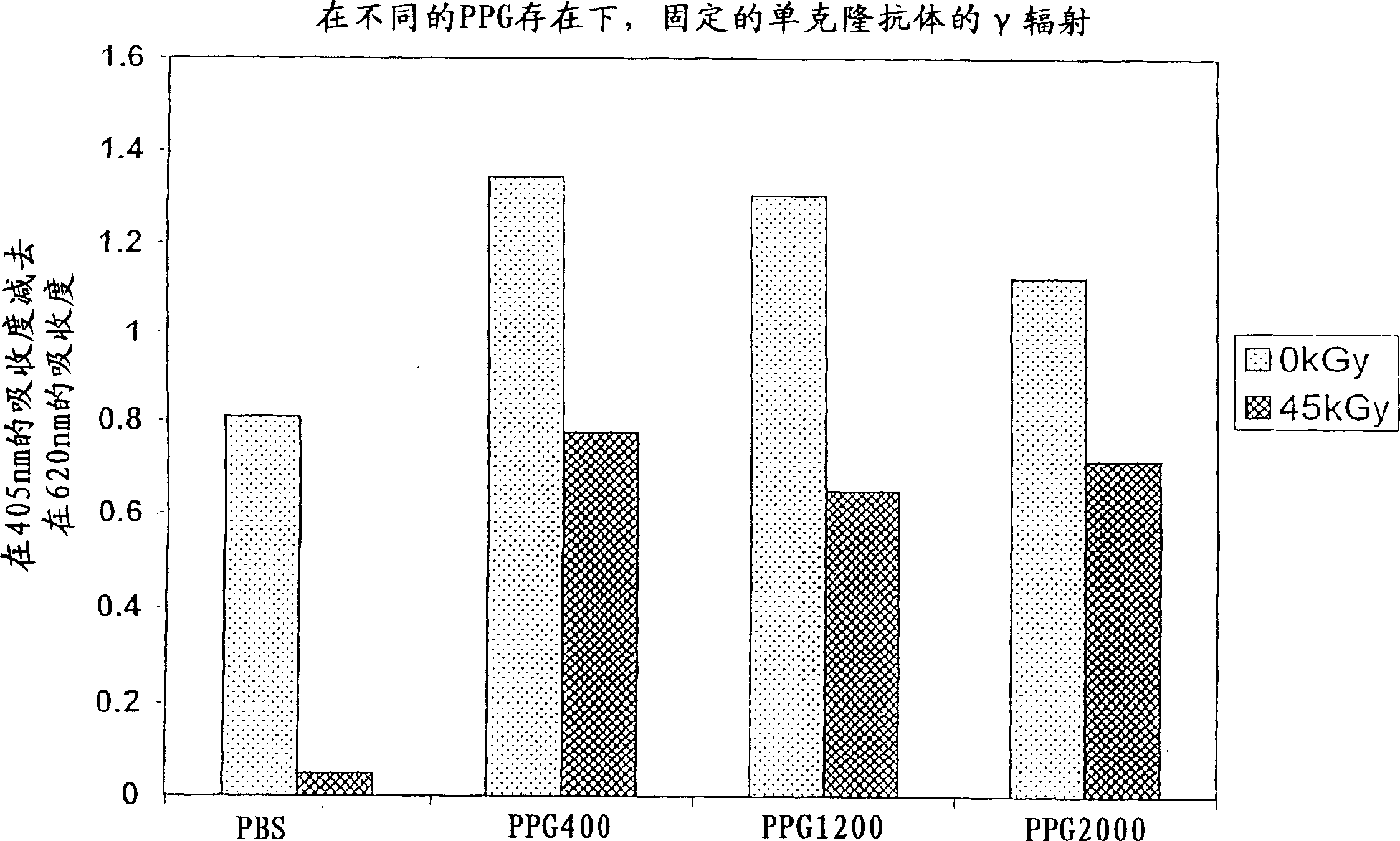
[0113] In this experiment, the activity (expressed by the ability to bind antigen) of immobilized anti-insulin monoclonal antibodies after irradiation in the presence of different forms of polypropylene glycol (molecular weight 400, 1200 and 2000) was determined.
[0114] method
[0115] In two 96-well microtiter plates (falcon plates - ProBind polystyrene cat. #353915), the wells were washed 4 times with full volume of PBS (pH 7.4). Once the two plates were prepared as described above, they were coated with 100 μl / well of freshly prepared 2 μg / ml anti-insulin in coating buffer and left overnight at 4°C. The plate was then briefly washed 3 times with PBS (pH 7.4), 100 μl of PPG400, PPG1200 or PPG2000 was added to specific wells, and each solution was made into 1 liter, ie 2-fold serial dilutions were performed with PBS. The two plates were tightly covered with a cover pad (Greiner cover pad cat. #381070 (USA Scientific)) and irradiated at 0 kGy / hour or 45 kGy (1.92 kGy / hour) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com