Pharmaceutical composition comprising factor vii polypeptides and pai-1 polypeptides
A composition, the technology of PAI-1, applied in the direction of drug combination, surgical drug, pharmaceutical formula, etc., can solve problems such as unsuccessful and difficult to treat
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0165] Compound preparation:
[0166] Preferably prepared by recombinant DNA techniques such as those described by Hagen et al. in Proc. Purified human Factor Vila suitable for use in the present invention.
[0167] Also by Broze and Majerus in J.Biol.Chem. 255(4):1242-1247, 1980 and Hedner and Kisiel in J.Clin.linvest., 71 : 1836-1841, Factor VII is produced by the method described in 1983. These methods yield Factor VII without detectable amounts of other coagulation factors. Even further purified Factor VII preparations can be obtained by including additional gel filtration as a final purification step. Factor VII is then converted into activated Factor Vila by known means, for example using several different plasma proteins, such as PAI-11a, IXa or Xa. On the other hand, as described by Bjoern et al. (Research Disclosure (Research Disclosure), 269, September 1986, pp. 564-565), by passing Factor VII through an ion exchange column, such as MonoQ (Pharmacia Fine Chemi...
Embodiment 1
[0218] Improved hemostatic clot stability by combining factor VIIa and PAI-1
[0219] method:
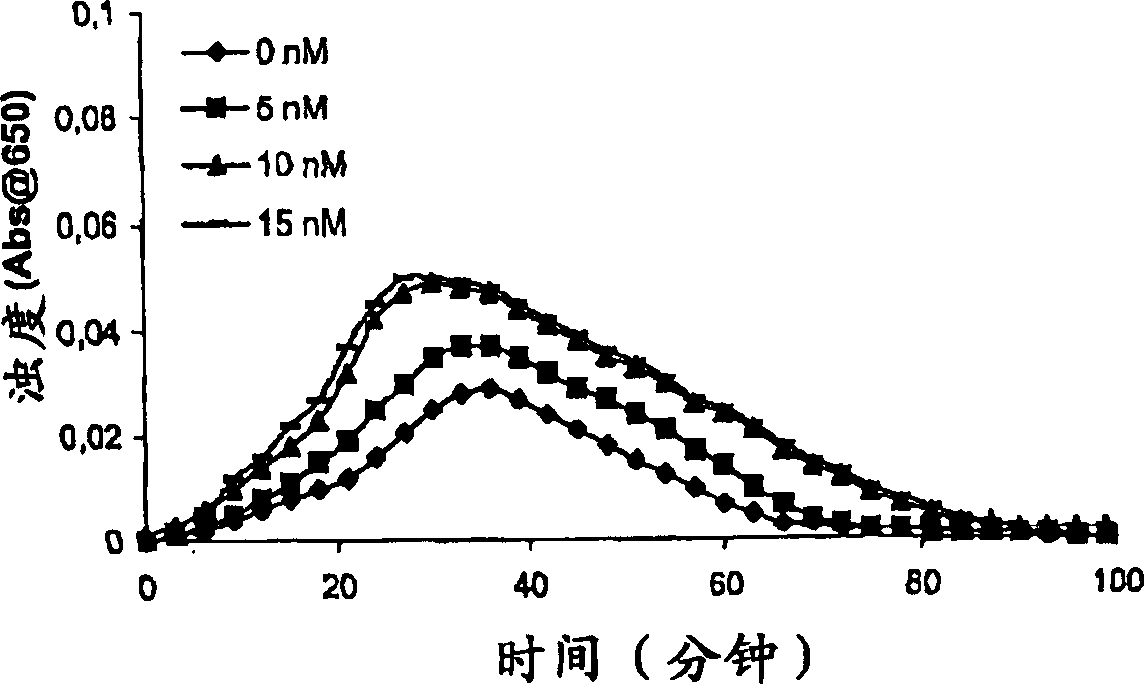
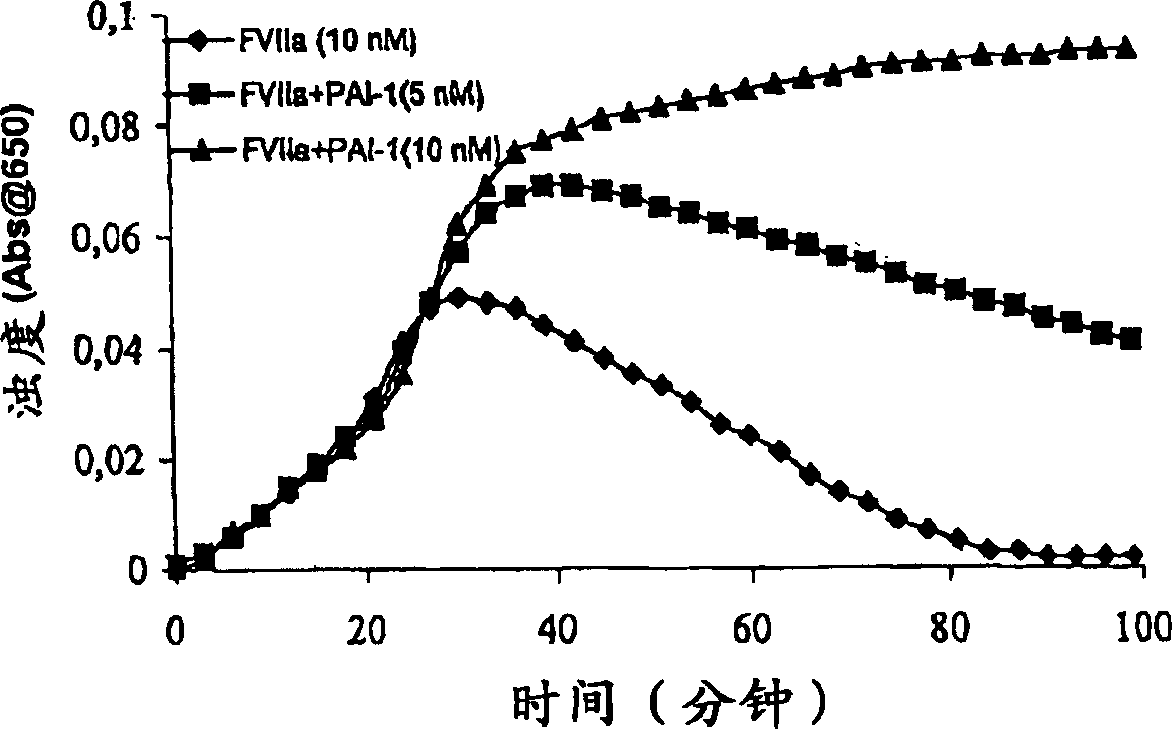
[0220] Clot lysis test: buffer (20 mM HEPES , 150mM NaCl, 5mM CaCl, pH7.4) Normal human plasma diluted 10-fold was added to a 96-well ELISA plate, and the turbidity at 650nm was measured at room temperature over a period of time. Where indicated, purified human PAI-1 (American Diagnostica, various concentrations) was included.
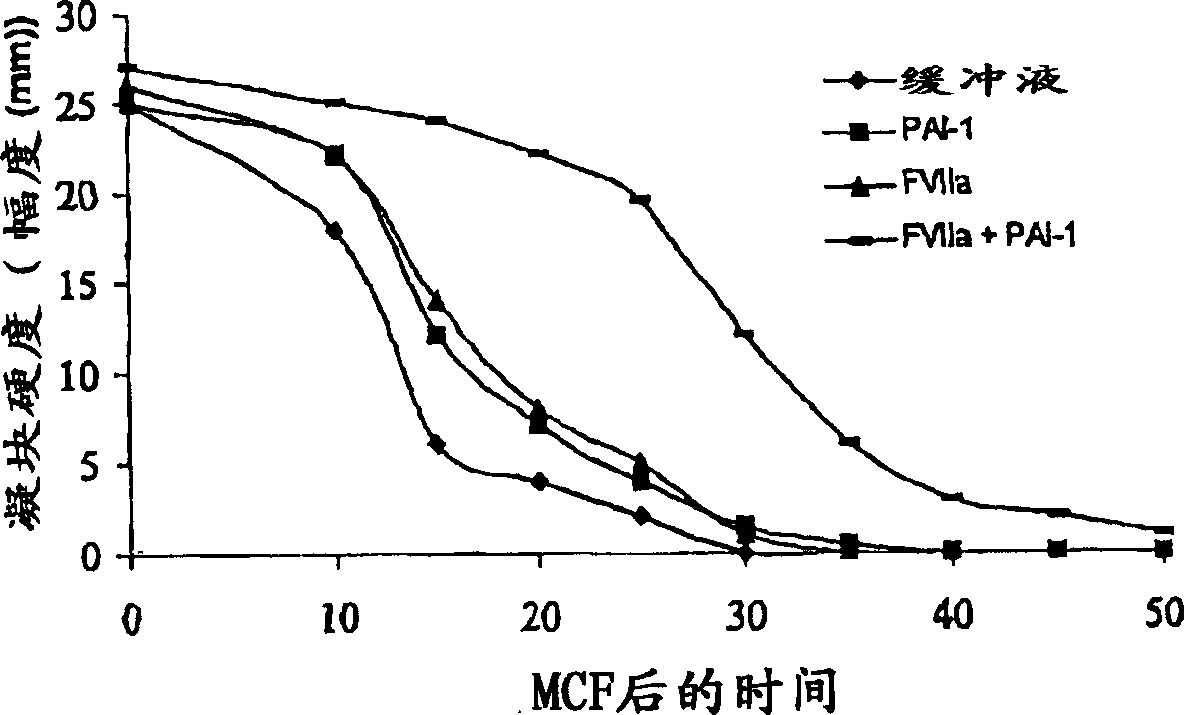
[0221] Rotational Thromboelastography (roTEG): Each assay was performed with citrated normal human plasma or Factor VIII-deficient plasma (George King Bio-Medical, Inc. #0800) spiked with 5 nM t-PA and analyzed separately Effect of 1 nM FVIIa or the addition of 1 nM FVIIa in combination with 30 nM PAI-1. Coagulation was initiated by the addition of Innovin (2000-fold diluted final concentration, DadeBehring #526945) and calcium (15 mM final concentration) in 20 mM HEPES, 150 mM NaCl, pH 7.4 buffer.
[0222] result:
[0223] Clot lysis test: Addition o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com