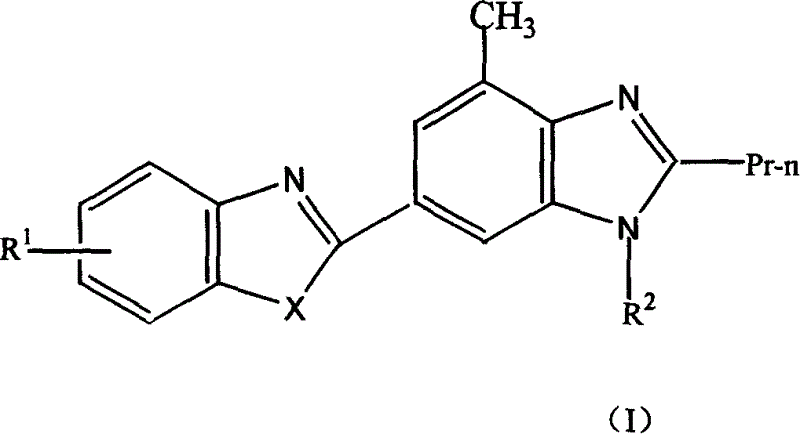
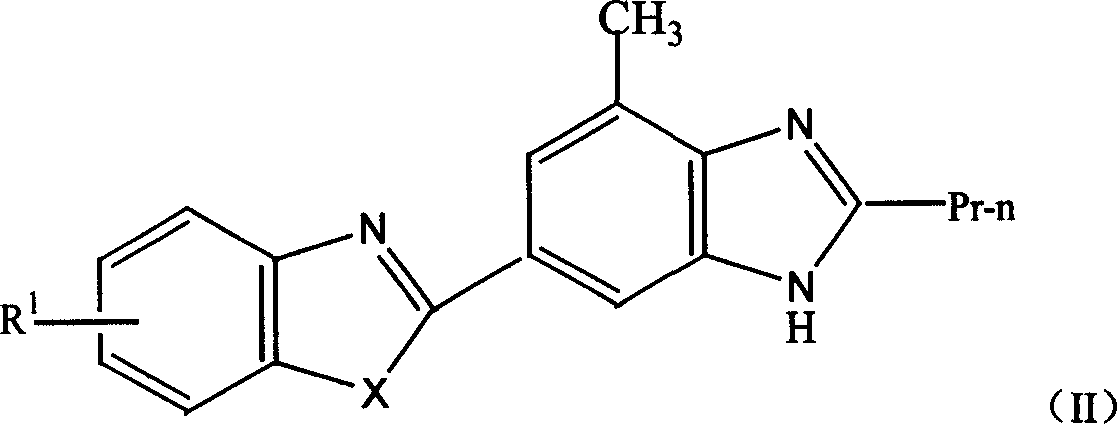
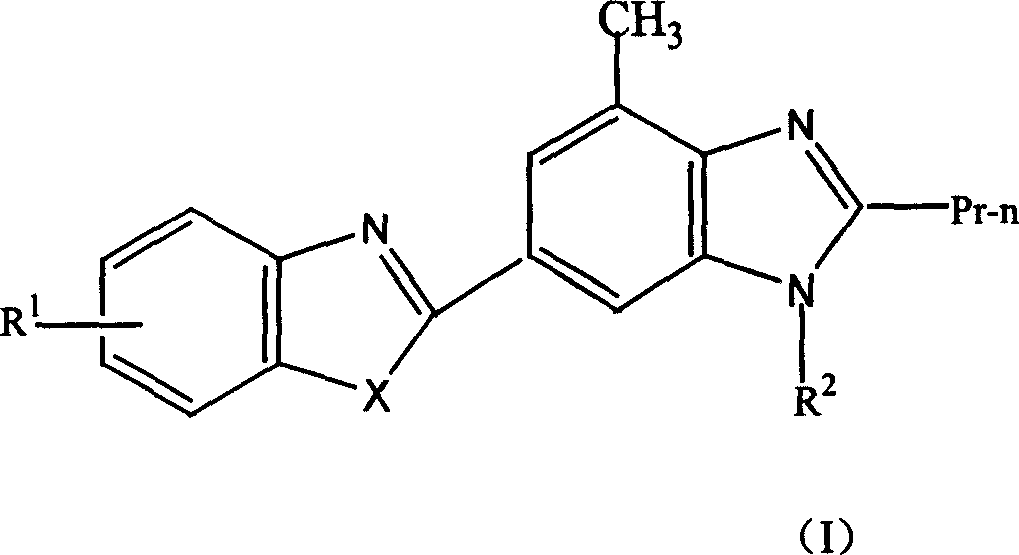
Novel medicine of non peptide like angiotensin II receptor antagonism agent, their preparation and application
A solvate and pharmaceutical technology, applied in the field of novel non-peptide angiotensin II receptor antagonist drugs, can solve the problems of large dosage and low activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0191] Example 1 3-methyl-4-nitro-benzoic acid methyl ester
[0192] Add compound Ia (1.81g), methanol (5ml) to the flask, then add concentrated H 2 SO 4 (0.5ml), after heating to reflux for about 2h, stop the reaction, cool to obtain a yellow solid, recrystallize the resulting yellow solid with methanol to obtain yellow needle-like crystal Ib (0.15g), the yield is about 74.3%, mp 78-79 ° C .1 H NMR (CDCl 3 , 500MHz) δ: 8.03 (br, 1H, Ar-H), 7.98 (m, 2H, Ar-H), 3.97 (s, 3H, CH 3 O), 2.63(s, 3H, PhCH 3 ).IR(KBr) v: 3005, 1740, 1620, 1590, 1520, 1350cm -1 .
Embodiment 2
[0193] Example 2 3-methyl-4-amino-benzoic acid methyl ester
[0194] The prepared Ib (0.20g) was dissolved in methanol (1.95ml), and about 0.1g of Raney nickel was added, followed by H 2 , after stirring at room temperature for about 4 h, the Raney nickel was filtered off, and the obtained filtrate was spin-dried to obtain a white solid Ic (0.15 g), the yield was about 92.1%, mp 181-121 ° C. 1 H NMR (CDCl 3 , 500MHz) δ: 7.76(s, 1H, Ar-H), 7.74(d, J=8.2Hz, 1H, Ar-H), 6.64(d, J=8.2Hz, 1H, Ar-H), 4.15( br,2H,NH 2 ), 3.85(s, 3H, CH 3 O), 2.17(s, 3H, PhCH 3 ).IR(KBr) v: 3470, 3380, 1700cm -1 .
Embodiment 3
[0195] Example 3 Methyl 4-(n-butylamido)-3-methylbenzoate
[0196] Add Ic (1.65g) in the there-necked flask, anhydrous MgSO 4 Dried CH 2 Cl 2 (7ml), redistilled triethylamine (4.3ml), then added dropwise 4.3ml of n-butyryl chloride, the temperature was controlled at 8°C to 10°C, stirred at room temperature for about 1h, suction filtered, and the filtrate was watered respectively (1.5ml×2) , saturated NaHCO 3 solution (1.5ml) and water (1ml×2), and the resulting organic phase was washed with anhydrous MgSO 4 After drying, the solvent was spun off to obtain a white solid, which was recrystallized from ethyl acetate to obtain Id (1.87g), with a yield of 79.6%, mp 125-127°C. 1 H NMR (CDCl 3 , 500MHz) δ: 8.37 (br, 1H, NH), 7.88 (m, 2H, Ar-H), 7.09 (s, 1H, Ar-H), 3.89 (s, 3H, CH 3 O), 2.41(t, J=7.3Hz, 2H, CH 2 CH 2 CH 3 ), 2.31(s, 3H, PhCH 3 ), 1.78 (m, 2H, CH 2 CH 2 CH 3 ), 1.04(t, J=7.4Hz, 3H, CH 2 CH 3 ).IR(KBr) v: 3300, 2950, 1730, 1670, 1530cm -1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com