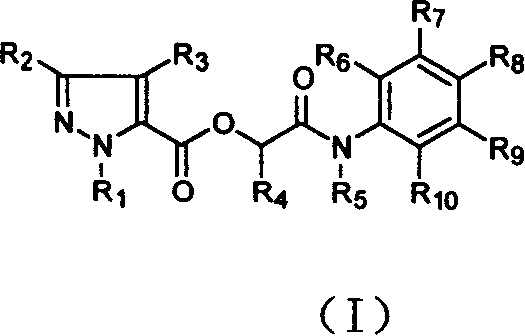
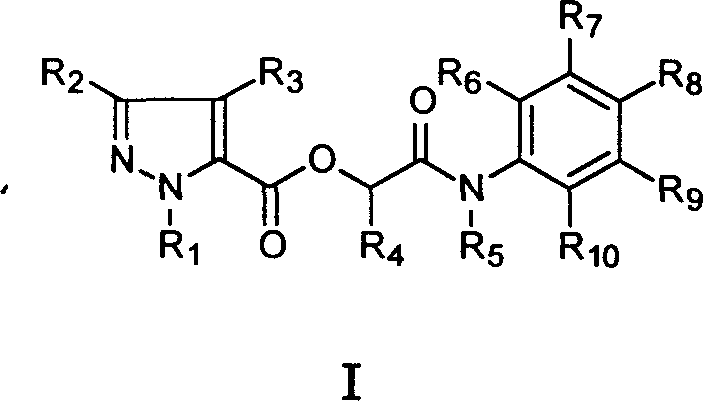
Compound in alpha (pyrazole formyloxy) acetanilide class of possessing fungicidal property
A technology of acetanilide and pyrazole carboxylic acid, applied in the fields of organic chemistry, biocides, animal repellents, etc., can solve the problem that the bactericidal activity has not been disclosed.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
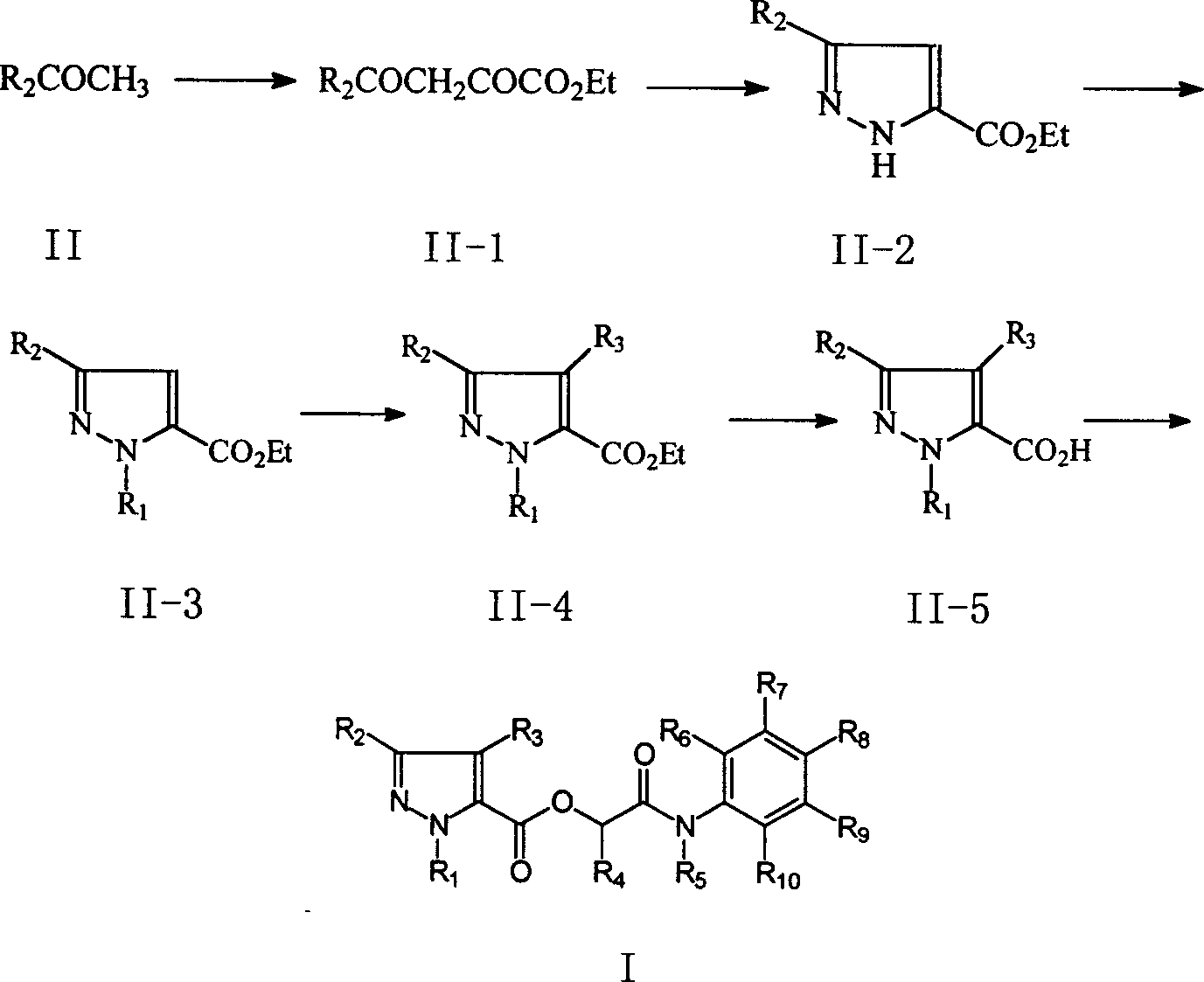
Examples
Synthetic example
[0064] Synthesis of Compound 41 in Table 1:
[0065]
[0066] A1 A2 B1
[0067] Add A1 (15 grams, 0.259 moles) in 500 milliliters of reaction flasks, sodium methylate (58 grams, 28%, 0.300 moles) and 100 milliliters of anhydrous methanols, under water-bath stirring, dropwise add A2 (36.7 grams, 0.251 moles) 50 ml of methanol solution was dropped in 4 hours, the water bath was removed, and the reaction was continued for 2 hours at room temperature. Under ice-water cooling, 200 milliliters of water and 200 milliliters of dichloromethane were added to the reaction solution, then the pH value was adjusted to 3 with concentrated hydrochloric acid, the layers were separated, the aqueous layer was extracted with 200 milliliters of dichloromethane, the organic layers were combined, and the organic layer was Wash with 200 ml of saturated aqueous sodium chloride, dry over anhydrous magnesium sulfate, and precipitate under reduced pressure to obtain 24 g of yellow oil B1.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com