Electrochemical cell for metal production
A metal and battery technology, applied in the field of electrochemical batteries for metal production, can solve the problems of producing by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
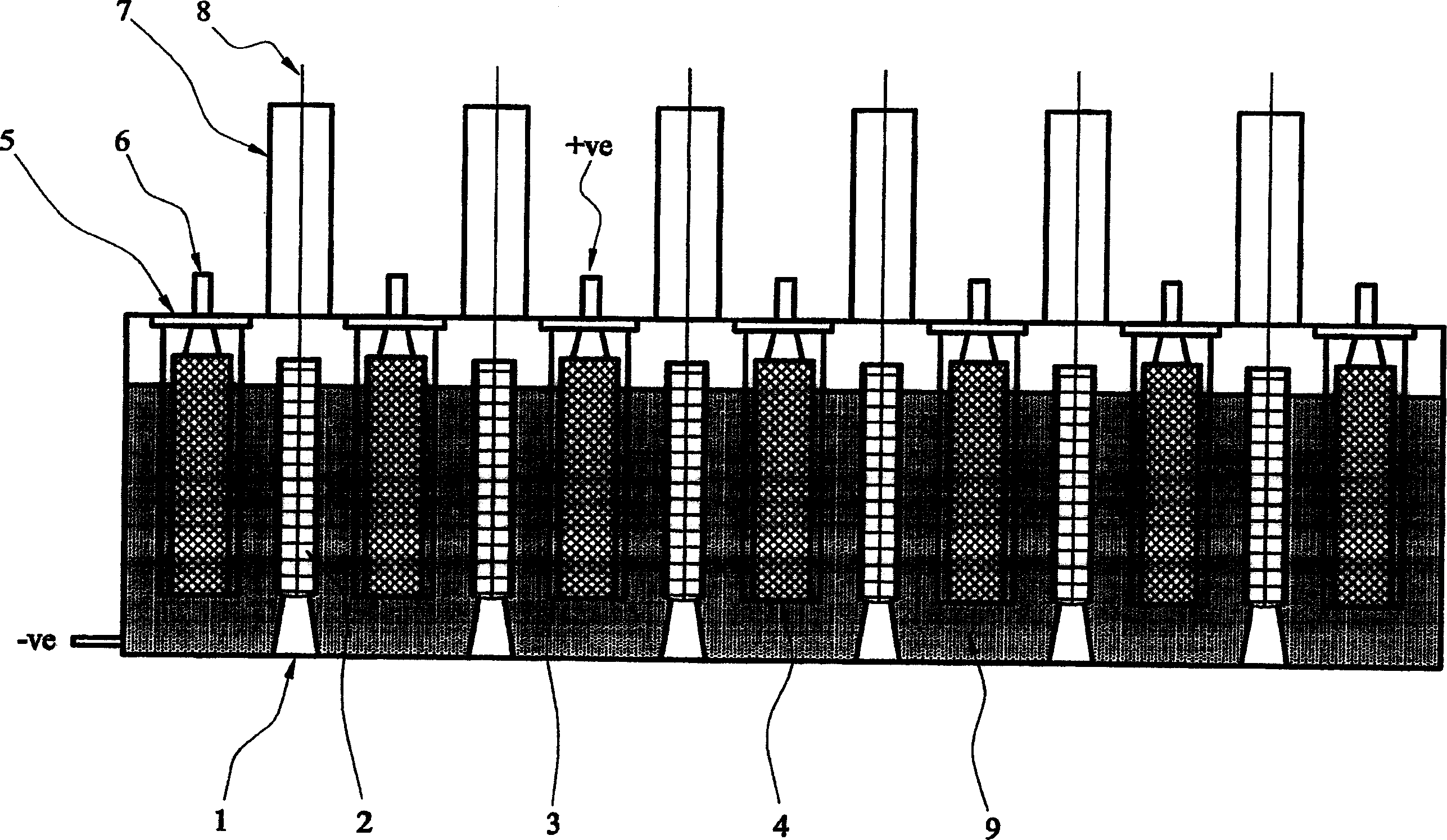
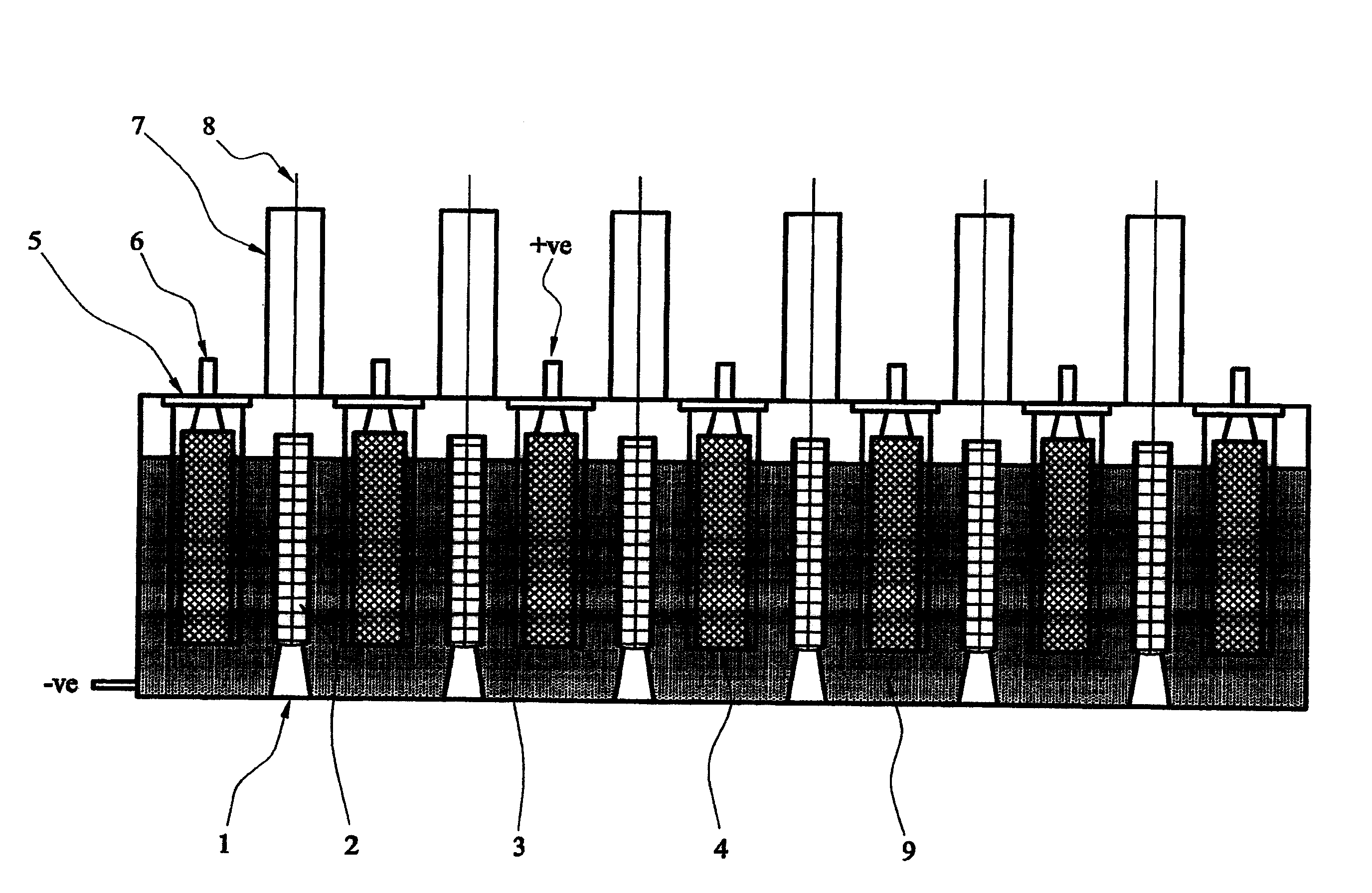
[0022] To practice an embodiment of the second aspect of the invention, an electrolytic cell was assembled using a metal oxide comprising spent nuclear fuel, having a carbon anode, a mesh cathode basket and a cathode rail post connector welded to the base of the cell. Radiation oxide fuel is placed in mesh baskets. The electrolyte consists of a molten salt or a mixture of such salts, such as CaCl 2 or BaCl 2 of chloride salts. A voltage is applied between the cathode and anode. The reaction at the cathode involves the diffusion of oxygen atoms to the solid surface, followed by ionization according to the following formula:
[0023]
[0024] The generated oxygen ions dissolve into the electrolyte and are transported to the anode where they are reoxidized to produce oxygen, CO or CO 2 gas. The cathodic potential can be controlled via a third reference electrode, thus ensuring that the reaction occurring at the cathode is the ionization of oxygen rather than the precip...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com