Process for producing nerve cells
A technology of nerve cells and neural stem cells, which is applied in the field of treatment of neurodegenerative diseases or nerve damage, and can solve problems such as being unsuitable for a large number of neural stem cells, difficult for neural stem cells, and slow in proliferation of neural stem cells.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
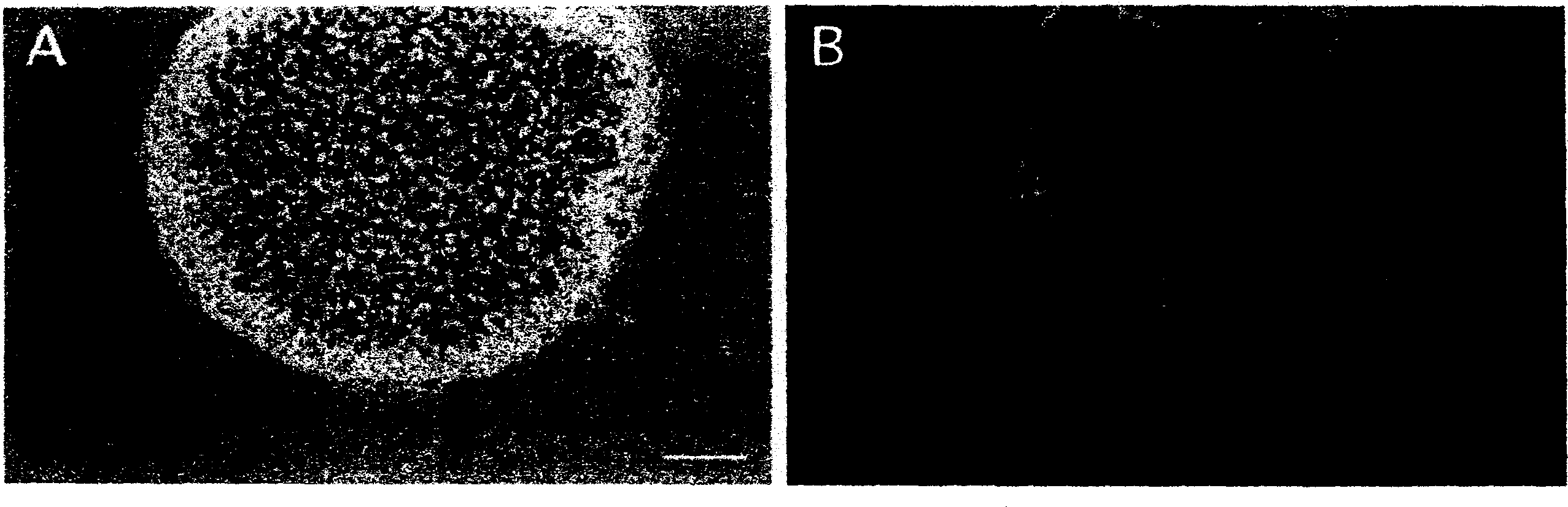
[0148] Example 1 Preparation of neural stem cells
[0149] As embryonic stem cells, an HK cell line (passage number 10 or less) established from an embryonic primordium of a C57BL / 6 mouse (day 3.5 after confirmation of a vaginal plug) was used by a conventional method. The aforementioned HK cells are cells with a small number of passages and are difficult to undergo spontaneous differentiation.
[0150] Fibroblasts prepared from syngeneic mice on day 14 of pregnancy were cultured to confluence in DMEM medium containing 10% (w / v) fetal calf serum (FCS). Then, mitomycin C (1 µg / ml) was added to the obtained cell culture, and 3 mice were incubated to obtain inactivated cells. Passivated cells were washed with phosphate-buffered saline and then trypsinized. The resulting cells were seeded on gelatin-coated plates to obtain feeder cell layers. A 60mm flat plate (1.5×10 6 cells / plate) and 4-well plates (3×10 5 cells / well).
[0151] Inoculate the above-mentioned HK cell lines o...
Embodiment 2
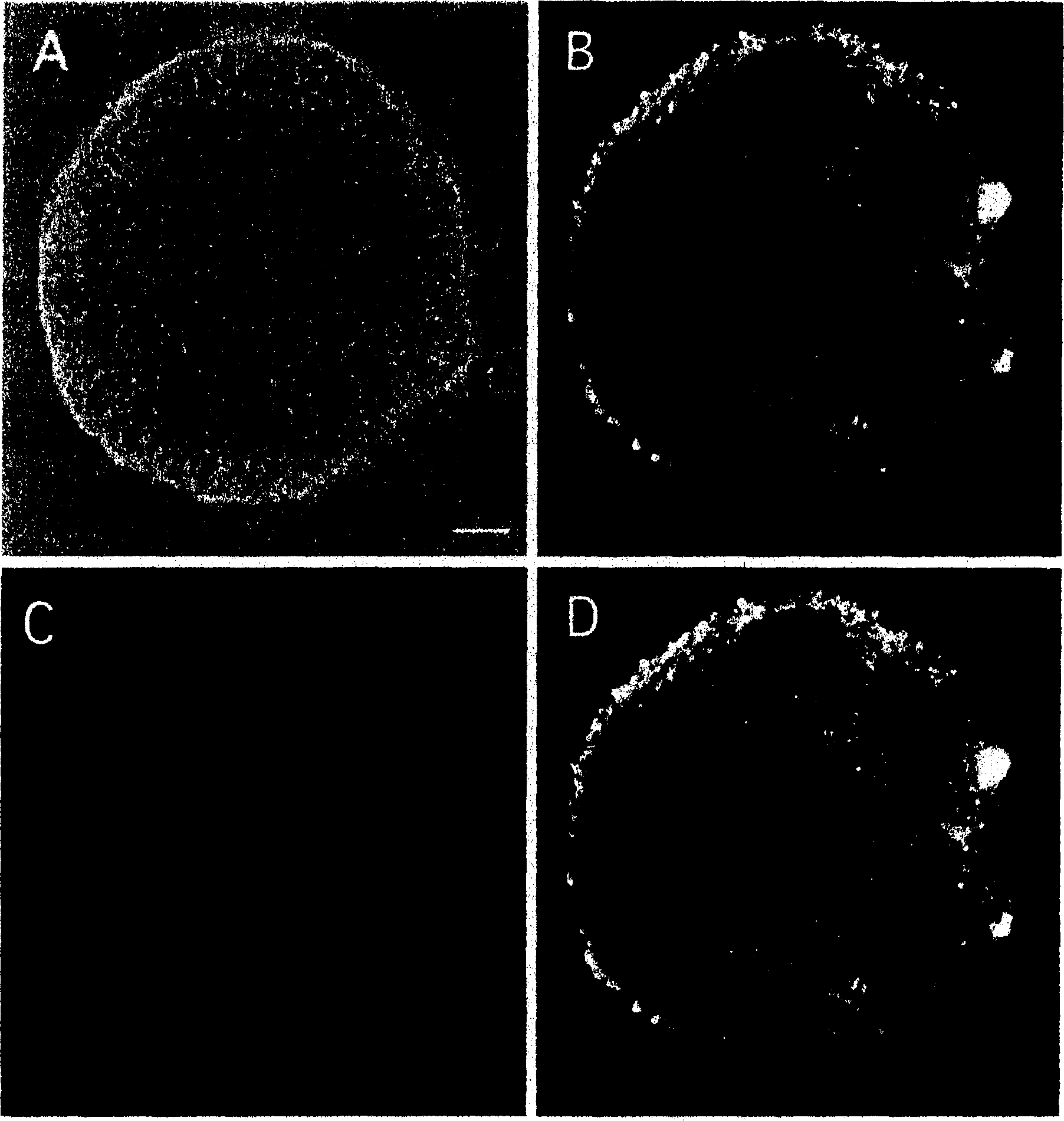
[0161] Example 2 Differentiation into nerve cells
[0162] Neural stem cells are differentiated and induced to the superficial SCS [SCS (suspension culture for 4 days) obtained in the above-mentioned embodiment 1] in a mixture of astrocyte conditioned medium and astrocyte basal medium (volume ratio 1: 1) in CO 2 In the incubator, 37°C, CO in the air 2 Suspension culture was carried out under 5% concentration and 100% humidified atmosphere.
[0163] In addition, in order to study the time-dependent change of the differentiation state in SCS, the suspension-cultured SCS was fixed in the same manner as in the above operation during 1 to 4-day culture.
[0164] As an indicator of differentiation into neurons, antibody TUJ1 that recognizes class III β-tubulin, a marker of weak neurons, was used. An upright fluorescent microscope (trade name: Eclipse E800) manufactured by Nikon was used for immunofluorescence histochemical observation.
[0165] As a result, it was observed in the i...
Embodiment 3
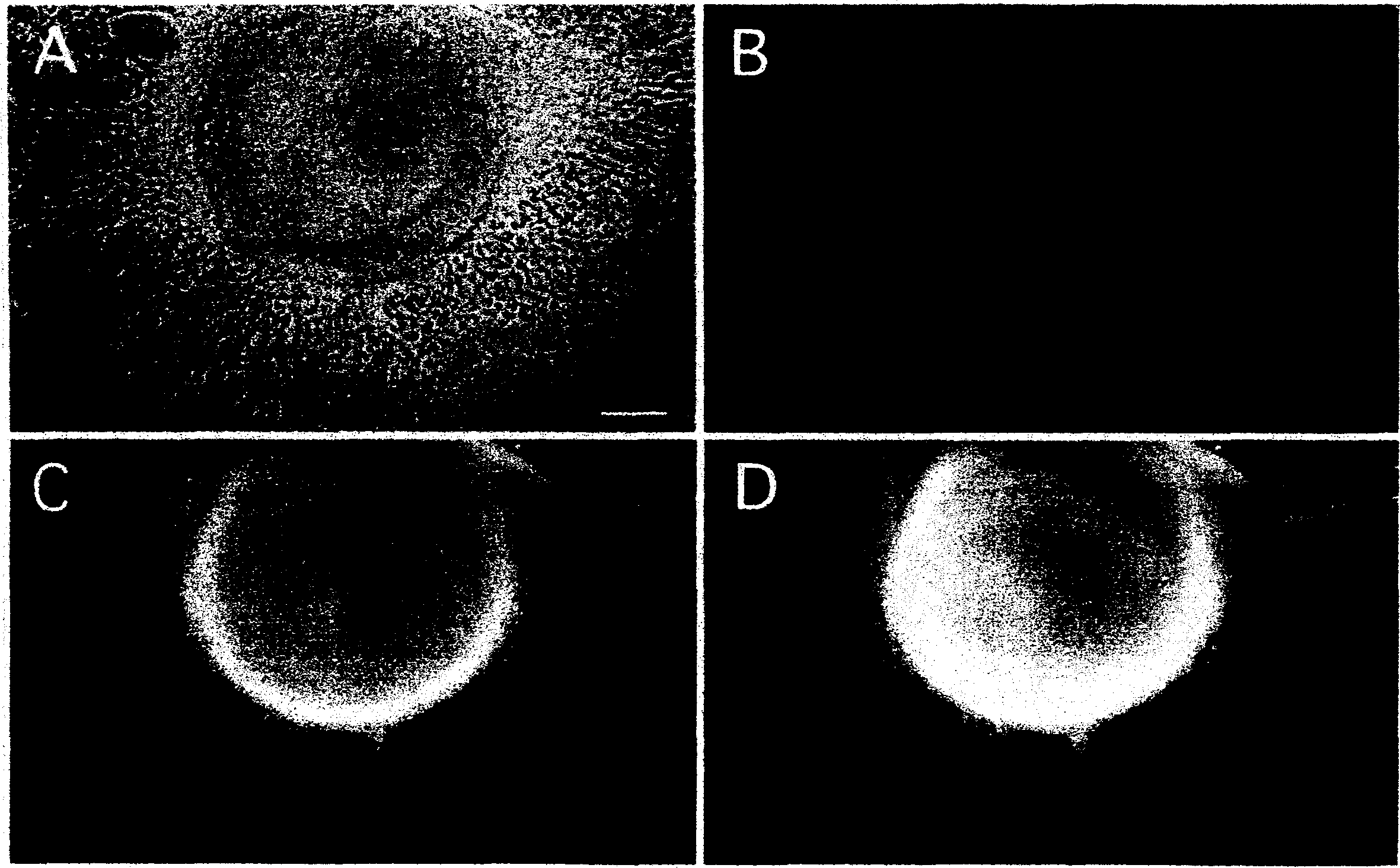
[0168] Example 3 Improvement of nerve cell differentiation method
[0169] By suspending culture of SCS in a mixture of astrocyte conditioned medium and astrocyte basal medium, neural stem cells can be prepared and induced to differentiate into immature neurons.
[0170] Next, the optimal culture conditions for the differentiation into neurons of the neural stem cells formed on the surface of the SCS were studied.
[0171] For the culture side surface of the culture dish coated with polylysine, further use 0.1mg / ml laminin or MATRIGEL diluted 10-20 times TM [manufactured by BD Bioscience] was treated to prepare an adhesive culture substrate. The 4-day suspension-cultured SCS was transferred to an adherent culture dish pre-added with astrocyte conditioned medium with a glass capillary tube, and stored in CO 2 In the incubator, 37°C, CO 2 Cultivate for several hours under 5% concentration and 100% humidified atmosphere. Results SCS adhered to the culture substrate.
[0172]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com