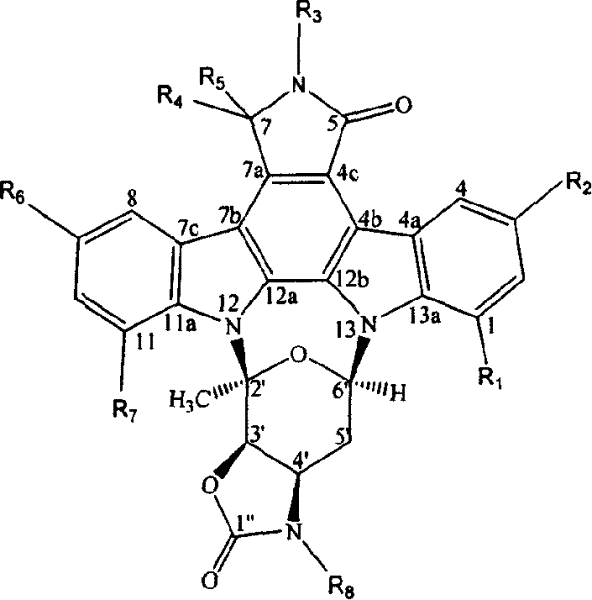
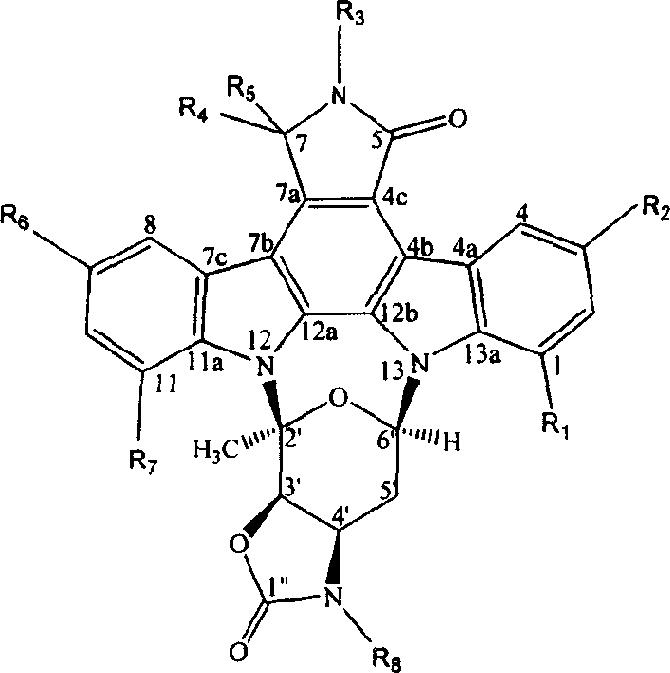
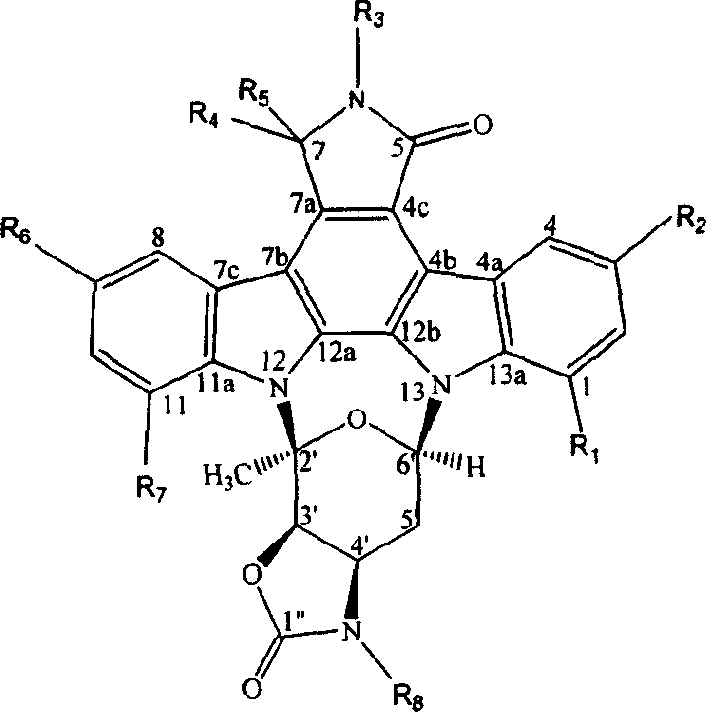
Indole carbazole alkaloid and its preparing method and use
A technology of alkaloids and uses, applied in the field of novel indolecarbazole alkaloid compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 Fermentative production and separation and purification of compound I
[0026] 1 Fermentation production
[0027] Fermentative culture of toxin-producing bacteria According to the conventional method of cultivating microorganisms, take an appropriate amount of Actinomadura sp.007 CCTCC M204076, inoculate it on the solid slant medium of Gaoshi Synthetic No. 1 agar, and cultivate it in an incubator at 28 degrees Celsius for 7 sky.
[0028] Get the appropriate amount of Actinomadura sp.007 CCTCC M204076 that the slant cultivated for 7 days, inoculate into one containing 100 milliliters of seed culture fluid (substratum composition: 20.0 grams of glucose, K 2 HPO 4 0.5 g, MgSO 4 0.5g, beef extract 3.0g, corn steep liquor 3.0g, yeast extract 10.0g, starch 10.0g, CaCO 3 2.0 g, 1 liter of artificial seawater, adjusted to pH 7.0) in an Erlenmeyer flask, cultured on a shaker at 28°C and 120 rpm for 48 hours to obtain a seed culture solution. Take the seed cultu...
Embodiment 2
[0035] Example 2 In Vitro Antitumor Activity Test of Compound I
[0036] 1 Experimental samples and experimental methods
[0037] Preparation of the test sample solution The test sample is the pure compound I isolated and refined in the above-mentioned Example 1. Accurately weigh an appropriate amount of sample, and prepare a solution with the required concentration with methanol for activity measurement.
[0038] Cell lines and subculture of cells Use human lung cancer A549 cells, human liver cancer BEL-7402 cells, human leukemia HL60 cells, mouse breast cancer tsFT210 cells and mouse leukemia P388 cells and other cancer cell lines. All kinds of cells were cultured in RPMI-1640 medium containing 10% FBS at 32°C (tsFT210 and P388 cells) or at 37°C (A549, HL60 and BEL-7402 cells) in an incubator filled with 5% carbon dioxide. subculture.
[0039] Cell Proliferation Inhibitory Activity Test Method
[0040] Human lung cancer A549 cells, human liver cancer BEL-7402 cells or mo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com