Pig tyraminase beta protein coding sequence
A technology of porcine monoamine oxidase and dog monoamine oxidase, which is applied in the field of protein coding sequence, can solve the problem of cloning and obtaining the coding protein sequence of porcine MAO-B gene.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Cloning of porcine MAO-B gene cDNA sequence
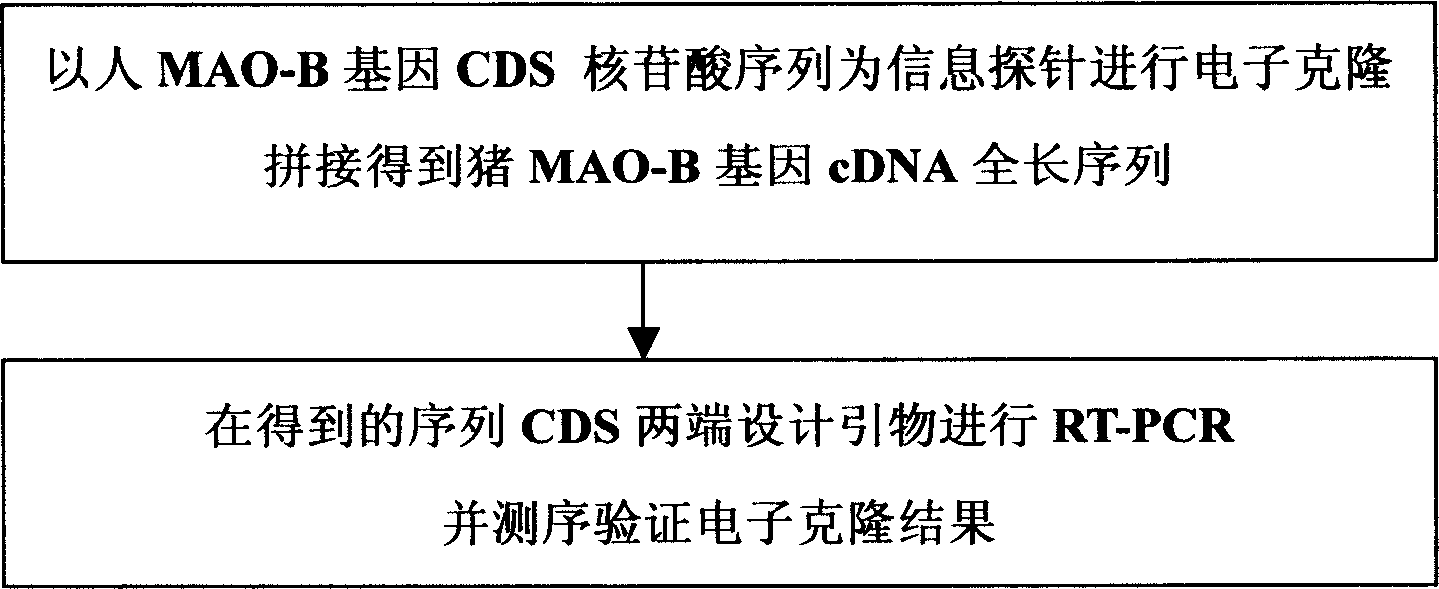
[0017] The cDNA cloning process of MAO-B gene is shown in the appendix figure 1 .
[0018] The specific operation method of the experimental method used in the cloning process is as follows:
[0019] 1. Electronic cloning
[0020] Using the human MAO-B gene CDS nucleotide sequence as the information probe, run the BLASTn program in NCBI to search in the pig EST database, and obtain a series of pig EST sequences of different lengths that are highly consistent with the probe, and then these sequences Perform assembly and connection to obtain a long sequence, and then use the obtained long sequence as a probe to perform BLASTn again in the pig EST database to obtain more EST sequences, so that the sequence is extended to both ends, and finally the obtained The sequences were spliced to obtain the complete sequence.
[0021] 2. Biological clone verification
[0022] (1) Isolation
[0023] The 30-day-old Dudu piglets were...
Embodiment 2
[0047] Homology Comparison of Porcine MAO-B Coding Sequence
[0048] Search and obtain human, bovine, mouse and dog MAO-B gene coding protein sequences on GenBank, and sequence it with the SEQ ID NO.1 sequence obtained by the present invention obtained by cloning and sequencing, and perform sequence alignment on the molecular biology software DNAman source comparison. The comparison results showed that the homology between SEQ ID NO.1 sequence and the MAO-B gene CDS sequence of human, bovine, mouse, dog and other species was 92.73%, respectively, and the homology of amino acid sequence was 93.81%.
[0049] The sequences and symbols involved in the present invention are listed as follows:
[0050] Information on SEQ ID NO.1
[0051] Shanghai Jiao Tong University
[0052] Porcine MAO-B protein coding sequence
[0053] 2
[0054] 1
[0055] 2559
[0056] DNA
[0057] pig (sus scrofa)
[0058]
[0059] CDS
[0060] (129)...(1691)
[0061] 1
[0062] cgccctgggc gg...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com