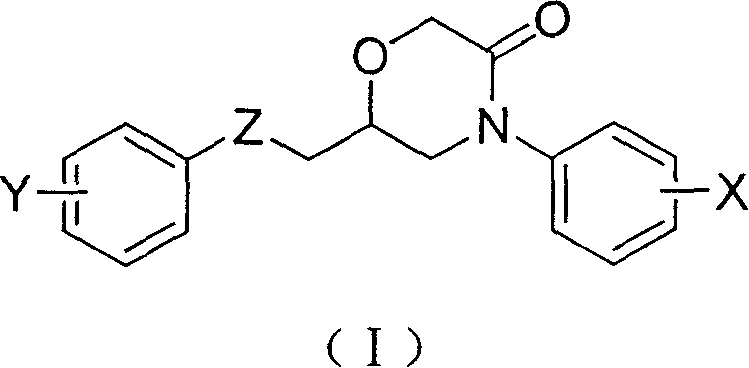
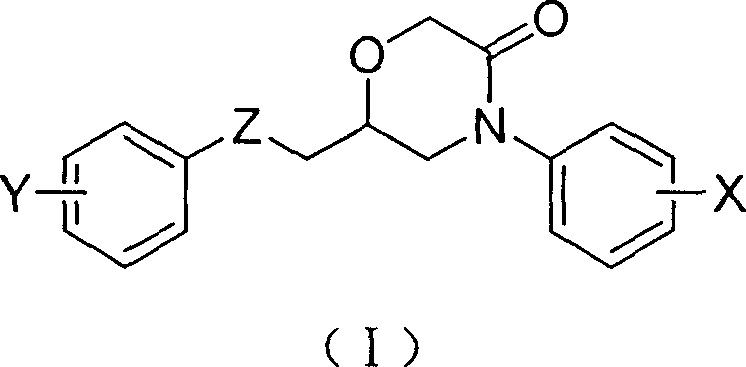
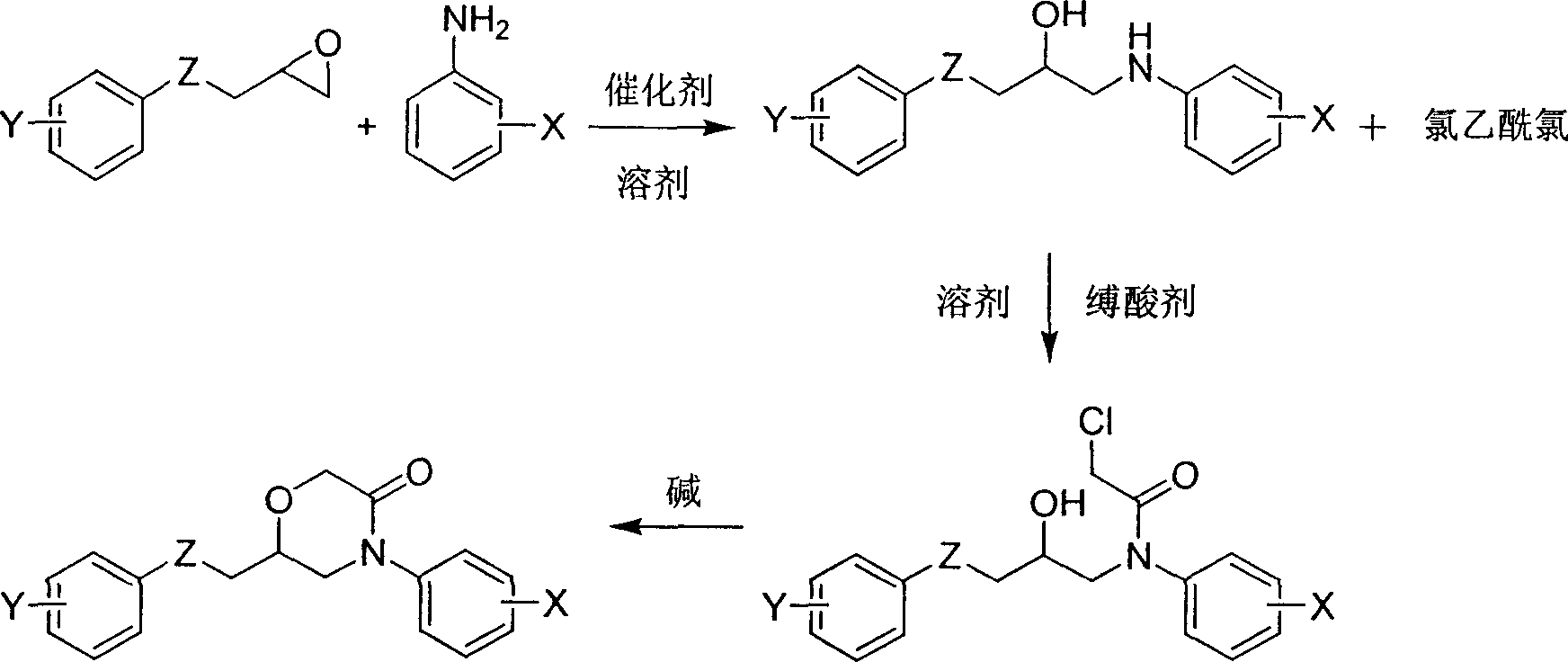
6-aryloxymethyl-4-aryl-3-morpholone derivative and its preparation method
A technology of methyl and alkoxy groups, applied in the field of 3-morpholinone derivatives and their preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: Preparation of 6-phenoxymethyl-4-phenylmorpholin-3-one
[0033] 1) Add 3 g (0.020 mol) of 3-phenoxy-1,2-epoxypropane into 30 ml of tetrahydrofuran, then add 2.05 g (0.022 mol) of aniline, then add 12 g of alumina and stir to reflux for 6 hours. Cool to room temperature, separate the alumina by filtration, evaporate tetrahydrofuran under elevated temperature and reduced pressure, and separate the residue by silica gel column chromatography (petroleum ether / ethyl acetate = 2:1, volume ratio) to obtain the aminoalcohol compound 1-benzene Oxy-3-anilino-isopropanol (2.43 g), 50% yield.
[0034] 2) 0.49 grams (0.002 moles) of 1-phenoxy-3-phenylamino-isopropanol was added to 5 milliliters of tetrahydrofuran, then 0.30 grams (0.0022 moles) of potassium carbonate was added, and then 0.25 grams (0.0022 moles) of ) chloroacetyl chloride, after continuing to react under ice bath for 2 hours, the tetrahydrofuran was distilled off under reduced pressure, the remaining ...
Embodiment 2
[0046] Embodiment 2: Preparation of 6-(4-chlorophenoxy)methyl-4-phenylmorpholin-3-one
[0047] 1) Add 3.69 grams (0.020 moles) of 3-(4-chlorophenoxy)-1,2-epoxypropane into 30 milliliters of tetrahydrofuran, then add 2.79 grams (0.030 moles) of aniline, then add 12 grams of alumina and stir Reflux for 6 hours. Cool to room temperature, separate the alumina by filtration, evaporate tetrahydrofuran under elevated temperature and reduced pressure, and separate the residue by silica gel column chromatography (developing solvent is petroleum ether / ethyl acetate=2:1, volume ratio) to obtain the aminoalcohol compound 1-( 4-Chlorophenoxy)-3-phenylamino-isopropanol (2.39 g), 43% yield.
[0048] 2) Add 0.56 grams (0.002 moles) of 1-(4-chlorophenoxy)-3-phenylamino-isopropanol to 5 milliliters of tetrahydrofuran, then add 0.32 grams (0.003 moles) of sodium carbonate, and then add 0.25 g (0.0022 moles) of chloroacetyl chloride, after continuing to react in an ice bath for 2 hours, the tet...
Embodiment 3
[0059] Embodiment 3: Preparation of 6-(4-nitrophenoxy)methyl-4-phenylmorpholin-3-one
[0060] 1) Add 3.9 grams (0.020 moles) of 3-(4-nitrophenoxy)-1,2-epoxypropane to 30 milliliters of tetrahydrofuran, then add 2.23 grams (0.022 moles) of aniline, and then add 12 grams of alumina Stir and reflux for 6 hours. Cool to room temperature, separate alumina by filtration, distill THF under reduced pressure, and separate the residue by silica gel column chromatography (developing solvent is petroleum ether / ethyl acetate=2:1, volume ratio) to obtain aminoalcohol compound 1-(4 -Nitrophenoxy)-3-anilino-isopropanol (3.52 g), yield 61%.
[0061] 2) Add 0.58 grams (0.002 moles) of 1-(4-nitrophenoxy)-3-phenylamino-isopropanol to 5 milliliters of tetrahydrofuran, then add 0.30 grams (0.0022 moles) of potassium carbonate, and then Add 0.25 g (0.0022 moles) of chloroacetyl chloride, continue to react under ice bath for 2 hours, distill off THF under reduced pressure, cool the remaining mixtur...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com