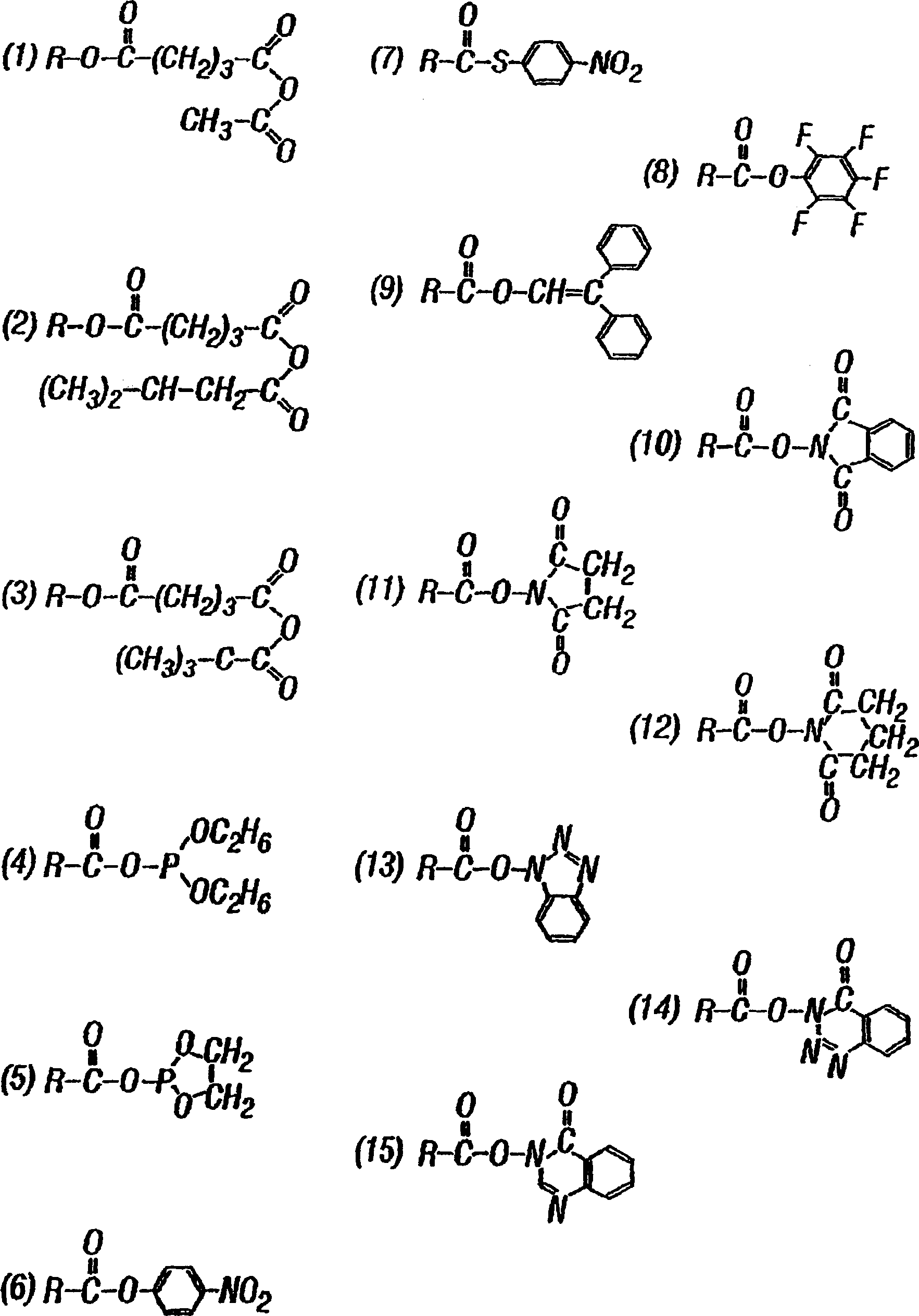Drug delivery from rapid gelling polymer composition
A composition and compound technology, applied in the direction of drug delivery, pharmaceutical formulation, etc., can solve problems such as increased tissue inflammation and deterioration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0605] Preparation of Two-Component Tissue Sealant Compositions
[0606] a. The first component
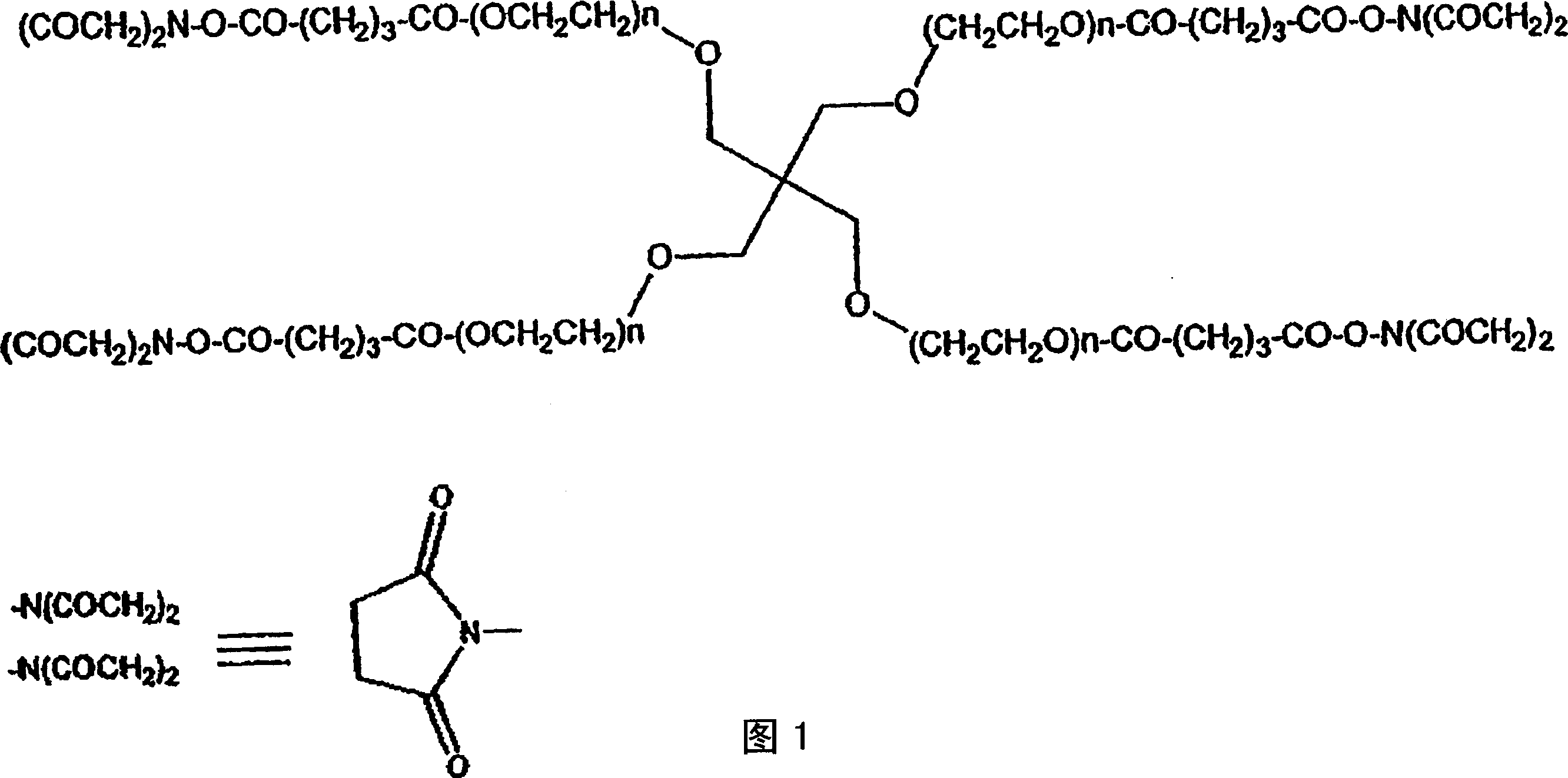
[0607] Pentaerythritol poly(ethylene glycol) ether tetra-succinimidyl glutarate ("SG-PEG") (molecular weight 10,000) was dissolved at a concentration of 20% w / v in 0.5 mM sodium phosphate pH 6.0. (Due to the susceptibility of the active ester to hydrolysis, this solution is not stable in aqueous media and should be used within one hour of preparation).
[0608] b. Second component
[0609] Pentaerythritol poly(ethylene glycol) ether tetra-mercapto (molecular weight 10,000) was dissolved in 300 mM sodium phosphate / sodium carbonate buffer ("P / C buffer") pH 9.6 at a concentration of 20% w / v. P / C buffer was prepared as follows: 300 mM sodium dihydrogen phosphate was mixed with 300 mM sodium carbonate to reach pH 9.6. The final molarity was approximately 117 mM phosphate and 183 mM carbonate. The solution is stable in aqueous media, but care should be taken to avoid e...
Embodiment 2
[0611] Surgical closure of animals
[0612] The right carotid artery of New Zealand white rabbits was exposed. The rabbits were treated with 200 U / kg of heparin and the vessels were clamped proximally and distally with atraumatic hemostatic clips. A puncture hole was made in the carotid artery using a 27G needle. Control rabbits were treated with tampon until hemostasis was achieved. For treated rabbits, approximately 0.5 mL each of the two components of the composition prepared as described in Example 1 was delivered to the defect site using a two-component nebulizer (Duo Flow, Hemaedics, Malibu, Calif.) point. After the material was in place for 30 seconds, the clamp was removed and the hemostasis time and blood loss were measured. The arteries of control rabbits were also kept in constant clamp for 30 seconds for consistency. The results are shown in Table 1.
[0613] Blood loss and hemostasis time as a function of treatment
[0614] The abo...
Embodiment 3
[0616] Surgical closure of ePTFE grafts
[0617] Dogs were treated with heparin to achieve an activated clotting time greater than 480 seconds. The dog's left iliac artery was exposed and isolated with proximally and distally placed atraumatic hemostatic clips. A 5 cm section of the artery was excised and replaced with an ePTFE (polytetrafluoroethylene) graft of the same diameter. The graft was deaired using a 27G needle prior to completion of anastamosis. The two components of the composition prepared according to Example 1 were delivered to the defect site at approximately 3.0 mL each using a two-component nebulizer (Cohesion Technologies, Inc., Palo Alto, Calif.). After the material was in place for 30 seconds, the clamp was removed and the hemostasis time and blood loss were measured. Except for material application, the procedure was repeated on the left iliac artery. The right iliac artery received tamponade only. The results are shown in Table 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com