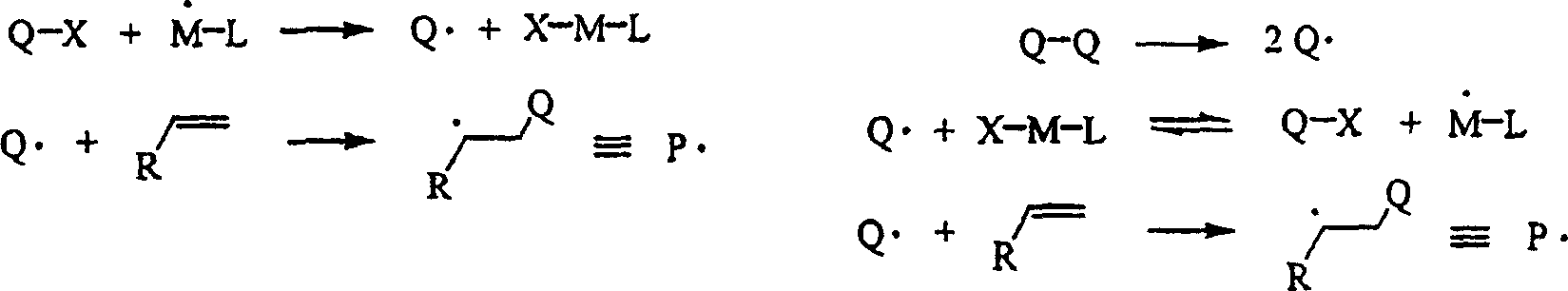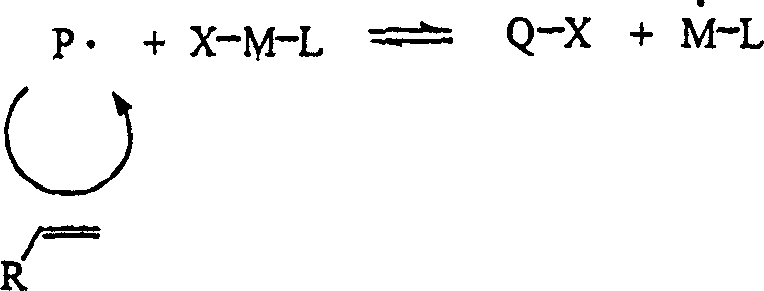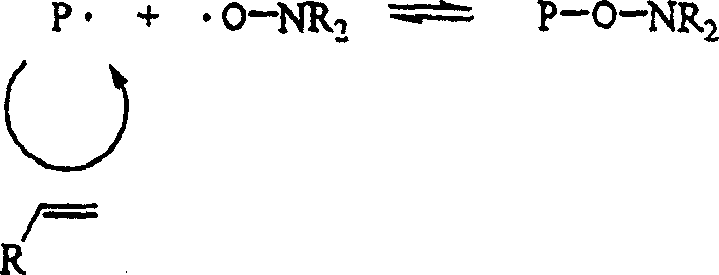Controlled polymerization
A control agent and polymer technology, applied in the field of controlled polymerization, can solve the problems of slow initiation speed, undesirable, long reaction time, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0184] Surfactant systems for in situ emulsification in styrene
[0185] A series of reactions were performed to identify potential surfactant / surfactant activator combinations for in situ emulsification. In a typical reaction, a solution of latent surfactant (~0.2 g) in styrene (5 mL) and a solution of surfactant activator in water (5 mL) were prepared and the aqueous solution was added to In styrene solution, an emulsion is produced. Selected latent surfactant / surfactant activator combinations to produce emulsions are summarized in the table below:
[0186] serial number
Embodiment 2
[0188] Control by RAFT methodM n Polymerization of polystyrene with PDI
[0189] A 2 gallon reaction vessel was first charged with 1,000 grams of styrene, 60.0 grams of oleic acid, and 7.2 grams of dibenzyl trithiocarbonate. The reactor was then purged with nitrogen, evacuated briefly, and charged with an aqueous solution containing 4,000 grams of RO water, 40.0 grams of potassium persulfate, 40.0 grams of tripotassium phosphate, and 16.4 grams of potassium hydroxide. As soon as the aqueous solution is mixed with the preceding organic material, a fine microemulsion is formed. The resulting mixture was then rapidly heated to 65°C. Complete conversion to a stable slightly yellow polystyrene latex occurred in less than 1.5 hours. The solids obtained after stripping were 20.6%. GPC analysis of the final polymer revealed that its M n at 54,000 with a PDI of 1.17.
Embodiment 3
[0191] Via the RAFT method using in situ generated polymerizable surfactants
[0192] Take Control M n Polymerization of polystyrene with PDI
[0193] A 2 gallon reaction vessel was initially charged with 1,000 grams of styrene, 60.0 grams of cetyl half ester of maleic acid and 7.2 grams of dibenzyl trithiocarbonate. The reactor was then purged with nitrogen, evacuated briefly, and charged with an aqueous solution containing 4,000 grams of reverse osmosis (RO) water, 40.0 grams of potassium persulfate, 40.0 grams of tripotassium phosphate, and 16.4 grams of potassium hydroxide. It should be noted that upon mixing the aqueous solution with the preceding organic material, a fine microemulsion is formed. The resulting mixture was then rapidly heated to 65°C. Complete conversion to a stable slightly yellow polystyrene latex occurred in less than 3 hours. The solids obtained after stripping were 23.7%. GPC analysis of the final polymer revealed that its M n ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| radius | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com