Alkaline button cell and method for producing same
一种碱性电池、制造方法的技术,应用在二次电池制造、干电池、电池电极等方向
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
[0025] In Embodiment 1, an example of the manufacturing method of the coin alkaline battery of this invention is demonstrated.
[0026] The manufacturing method of the button alkaline battery of this embodiment includes sealing the positive electrode container with the negative electrode sealing body through the gasket, and setting the negative electrode containing the negative electrode active material and the alkaline electrolyte in the sealed space thus formed.
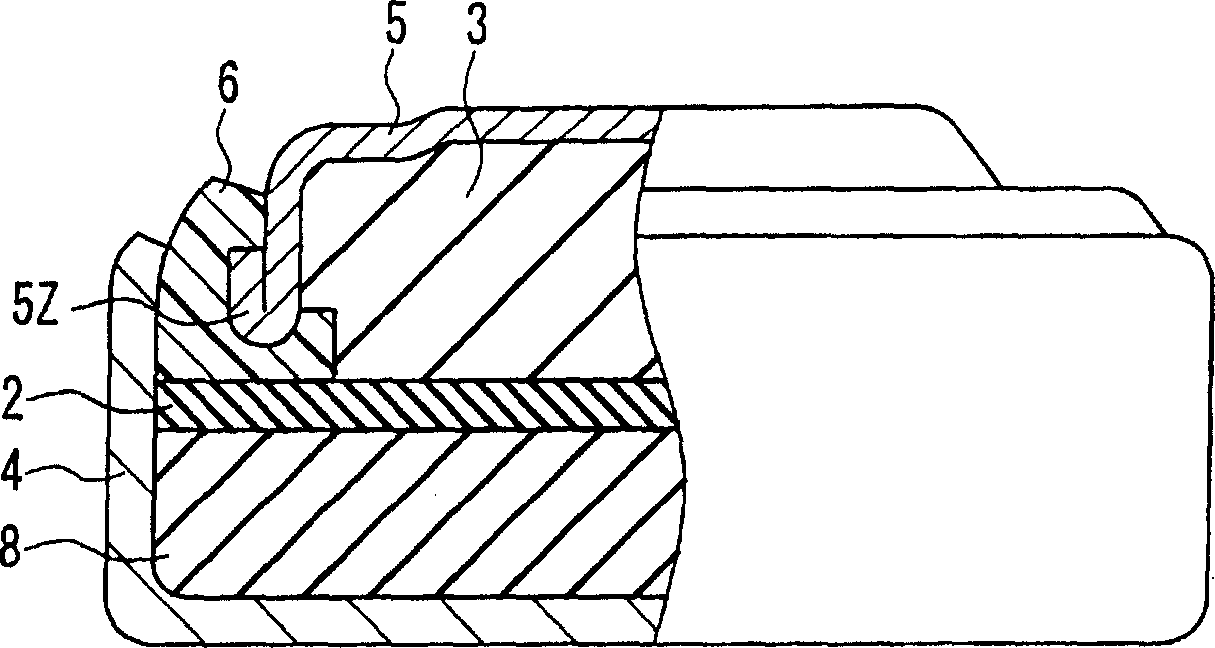
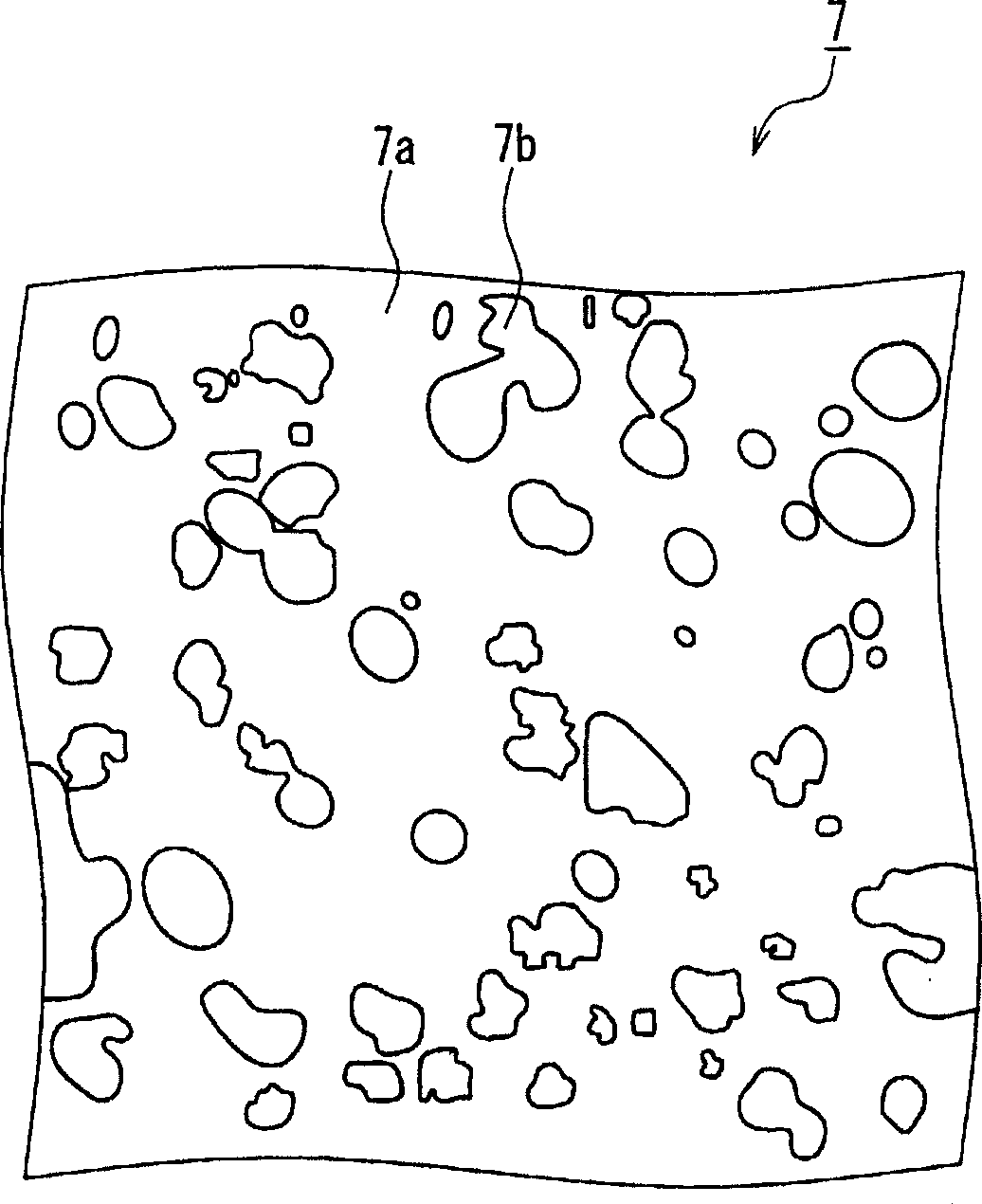
[0027] Such as figure 1 As shown, the manufacturing method of the button alkaline battery of this embodiment includes the following metal layer forming step, that is, in the state where the negative electrode 3 and the negative electrode sealing body 5 are arranged so that the negative electrode 3 and the negative electrode sealing body 5 are in contact, The negative electrode 3 and the negative electrode sealing body 5 are left for a predetermined period of time to form the metal layer 7 on the surface of the nega...
Embodiment approach 2
[0071] In Embodiment 2, the button alkaline battery manufactured by the manufacturing method of the button alkaline battery of Embodiment 1 is demonstrated.
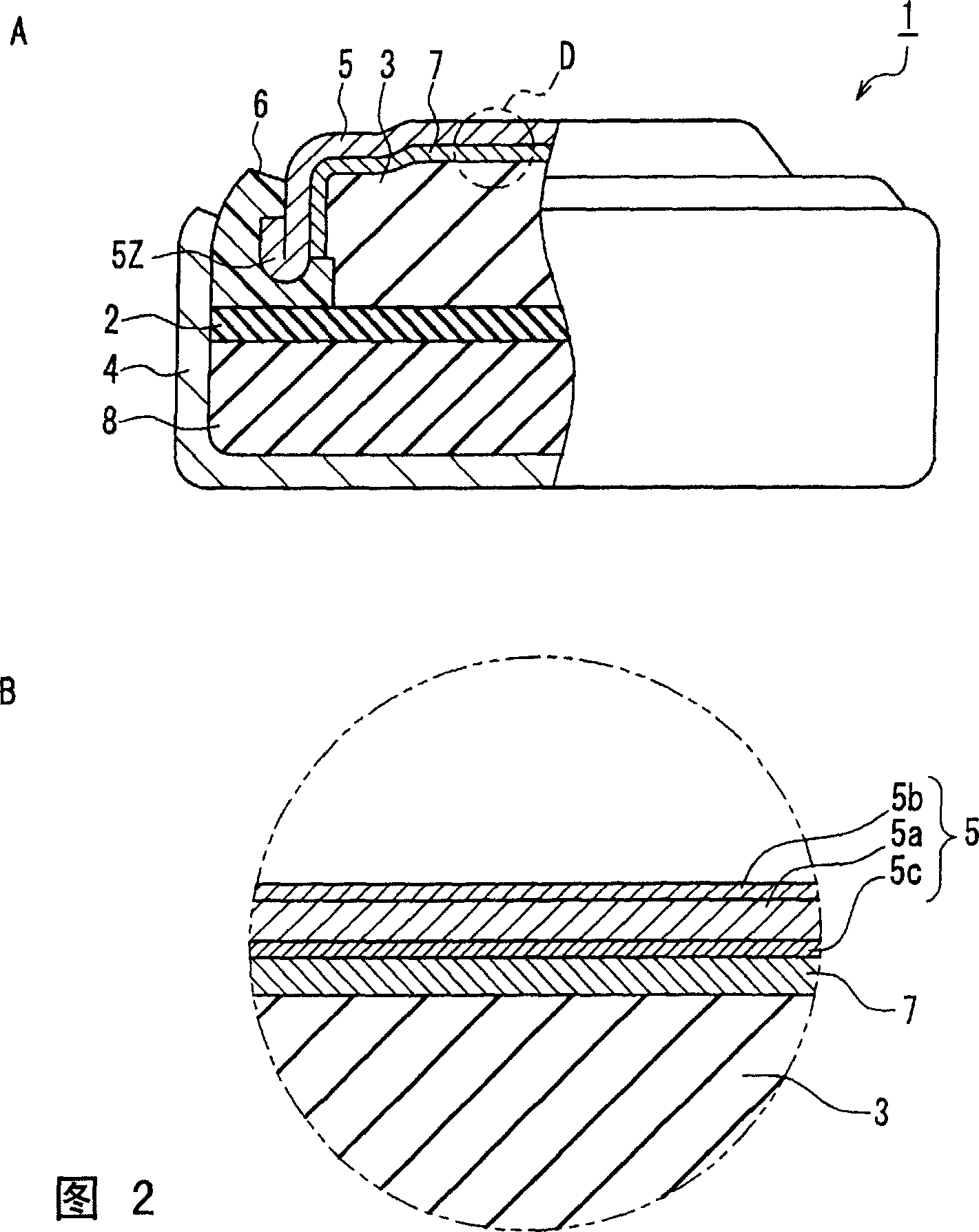
[0072] As shown in FIG. 2A, the button alkaline battery 1 of the present embodiment uses a negative electrode sealing body 5 to seal the positive electrode container 4 through the gasket 6, and in the closed space formed in this way, the negative electrode containing the negative electrode active material and the alkaline electrolyte is arranged. 3.
[0073]The negative electrode 3 contains, for example, a powdery negative electrode active material and an alkaline electrolyte. The negative electrode active material is composed of mercury-free zinc or mercury-free zinc alloy. The negative electrode 3 contains most of the alkaline electrolyte constituting the button alkaline battery.
[0074] The positive electrode 8 contains a molded body made of a positive electrode active material such as silver oxide, manganese dioxi...
Embodiment 1
[0092] (Preparation of Alkaline Electrolyte)
[0093] 550 g of sodium hydroxide (purity 96%), 100 g of zinc oxide (purity 99%), and 10 g of indium hydroxide were added to 340 g of water to obtain a mixed solution, which was stirred for 20 minutes (stirring speed: 1500 rpm). Next, 1010 g of water was further added to the above mixed solution, followed by stirring for another 20 minutes (stirring speed: 1500 rpm). The mixed solution obtained by the above method was left to stand at room temperature (25° C.) for 3 days, and thereafter, undissolved indium hydroxide was removed by taking out the supernatant liquid from the mixed solution. The upper clarified liquid was used as an alkaline electrolyte.
[0094] The concentration of indium in the alkaline electrolyte was analyzed using ICP (Inductively Coupled Plasma Luminescence Analyzer) ("IRIS 1000" manufactured by Japan Jarel Ash Co., Ltd.). The indium concentration in the alkaline electrolyte was 789 ppm. Hereinafter, the con...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com