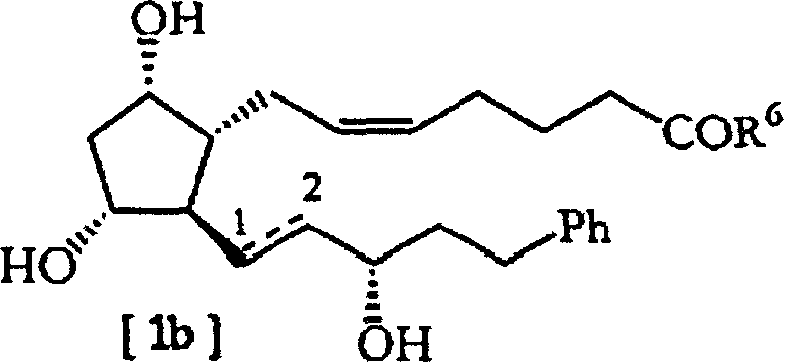
A new process for the preparation of 17-phenyl-18, 19, 20-trinor-PGF2A and its derivatives
A technology of prostaglandins and derivatives, applied in organic chemistry methods, active ingredients of anhydrides/acids/halides, organic chemistry, etc., can solve problems such as no recovery of by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
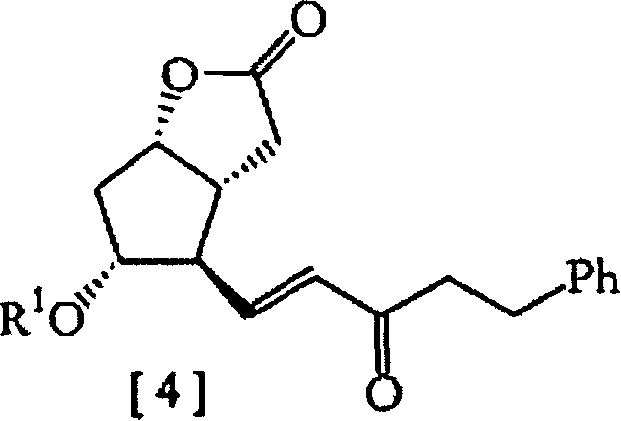
[0066] (3aR, 4R, 5R, 6aS)-hexahydro-4-(3-oxo-5-phenyl-1E-pentenyl)-5-(p-phenylbenzoyloxy)-2H-cyclopentyl [b]furan-2-one[4a]
[0067]
[0068] Wherein PPB is p-phenylbenzoyloxy.
[0069] Flowchart 6
[0070] 1) Preparation of dimethyl (2-oxo-4-phenylbutyl) phosphonate
[0071] 1.1) 1-Bromo-4-phenyl-2-butanone
[0072] A freshly prepared solution of bromine (258.9 g) in methanol (600 mL) was added dropwise to continuously stirred benzyl acetone in methanol (600 mL) at 7 to 10 °C during 1 h 20 min (222.3g) in solution. To maintain the necessary temperature (7 to 10 °C) because of the highly exothermic reaction, the flask should be immersed in an ice-water bath. After the bromine red color disappeared, water (1500 mL) was added to the mixture, and the resulting mixture was stirred overnight. The resulting organic layer (at the top) was separated and the aqueous phase was extracted with dichloromethane (2 x 600 mL). The combined organic layers ...
Embodiment 2
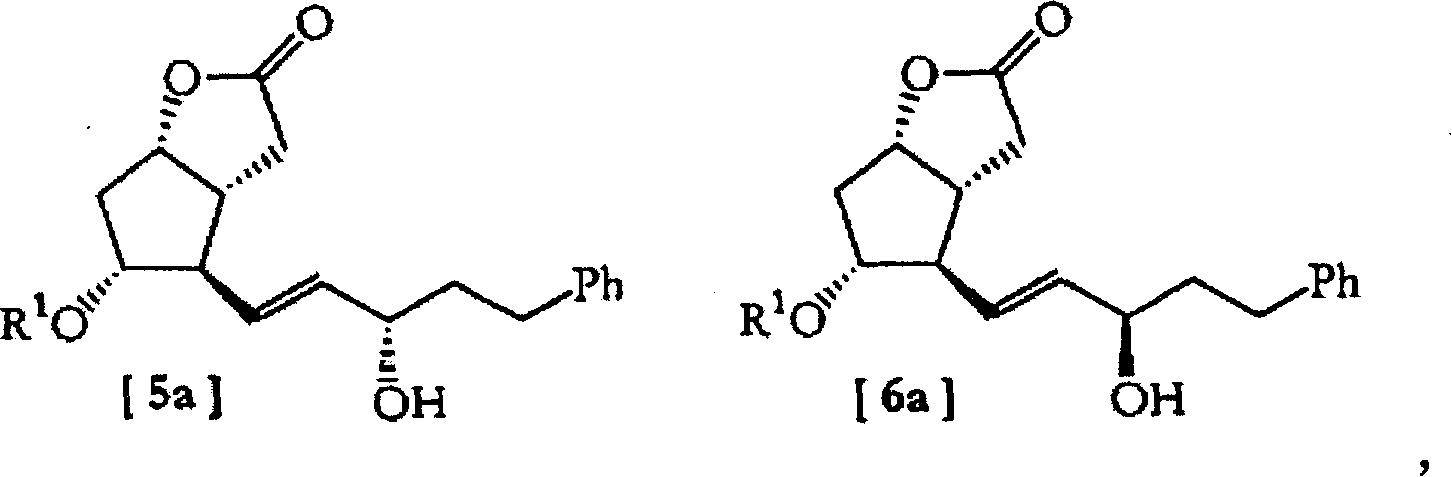
[0082] (3aR, 4R, 5R, 6aS)-hexahydro-5-(p-phenylbenzoyloxy)-4-[(3S)-5-phenyl-3-[(tetrahydro-2H-pyran- 2-yl) oxygen] -1E-pentenyl] -2H-cyclopentene [b] furan-2-one [5] (C 1 =C 2 , R 1 = PPB and R 2 =THP)
[0083]
[0084] Flowchart 7
[0085] 1) (3aR, 4R, 5R, 6aS)-hexahydro-4-[(3S) and (3R)-3-hydroxy-5-phenyl-1E-pentenyl]-5-(p-phenylbenzyl Acyloxy)-2H-cyclopenta[b]furan-2-one[5a](R 1 =PPB) and [6a](R 1 =PPB)
[0086] Prepared by dissolving (-)-B-chlorobis(dimethylmethyleneoxybicyclooctyl)camphenylborane (26.0g) in THF (150mL) at -23 to -25°C The solution of was added dropwise to a stirred solution of compound [4a] (26.0 g) dissolved in THF (250 mL). The resulting mixture was stirred at this temperature for 8 hours (TLC monitoring), then the reaction was quenched by adding 30 mL of methanol at -23 to -25 °C. The temperature of the resulting solution was raised to room temperature, and stirred at this temperature for 14 hours. The mixtur...
Embodiment 3
[0100] (3aR, 4R, 5R, 6aS)-hexahydro-5-(p-phenylbenzoyloxy)-4-[(3R)-5-phenyl-3-[(tetrahydro-2H-pyran- 2-yl) oxygen] pentyl] -2H-cyclopenta[b] furan-2-one [5] (C 1 -C 2 , R 1 = PPB and R 2 =THP)
[0101]
[0102] Wherein PPB is p-phenylbenzoyl and THP is tetrahydro-2H-pyran-2-yl.
[0103] Flowchart 8
[0104] Compound [5] (C 1 =C 2 , R 1 = PPB and R 2 = A mixture of THP) (80.0 g), palladium on carbon catalyst (16 g) and ethyl acetate (1.0 L) for 3 hours. The reaction mixture was then filtered and evaporated under reduced pressure. The resulting oily residue was crystallized from a 4:1 (volume ratio) mixture of hexane and ethyl acetate to give 71.4 g (89% yield) of compound [5] (C 1 -C 2 , R 1 = PPB and R 2 =THP), its melting point is 103~105℃, [α] D 20 is -107° (c 1.0, MeCN). 1 HNMR (CDCl 3 )δ: 8.03(d, J=8Hz, 2H); 7.60-7.67(m, 4H); 7.36-7.48(m, 3H); 7.14-7.24(m, 5H); 5.20-5.30(m, 1H); 5.00-5.15 (m, 1H); 4.50-4.70 (m, 1H); 3.89-3.95 (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com