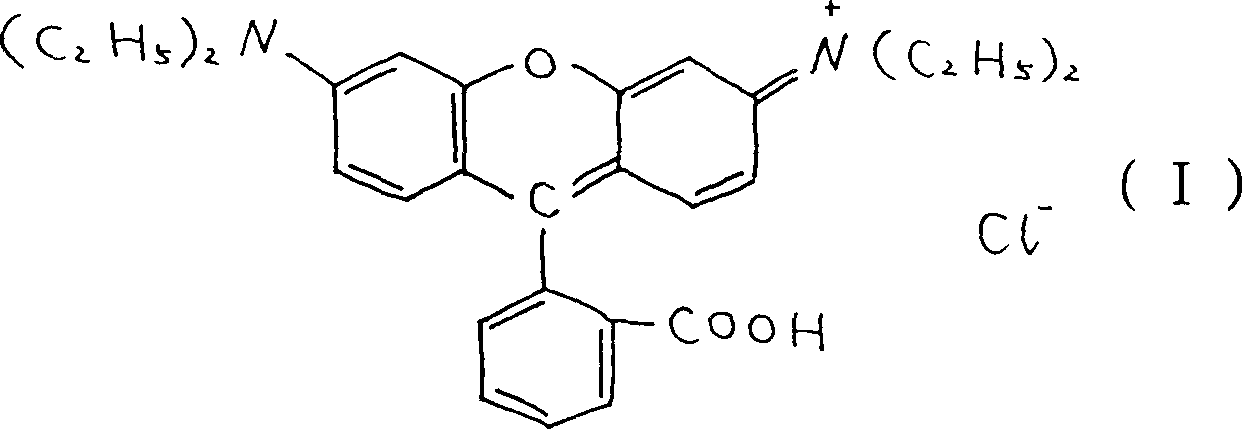
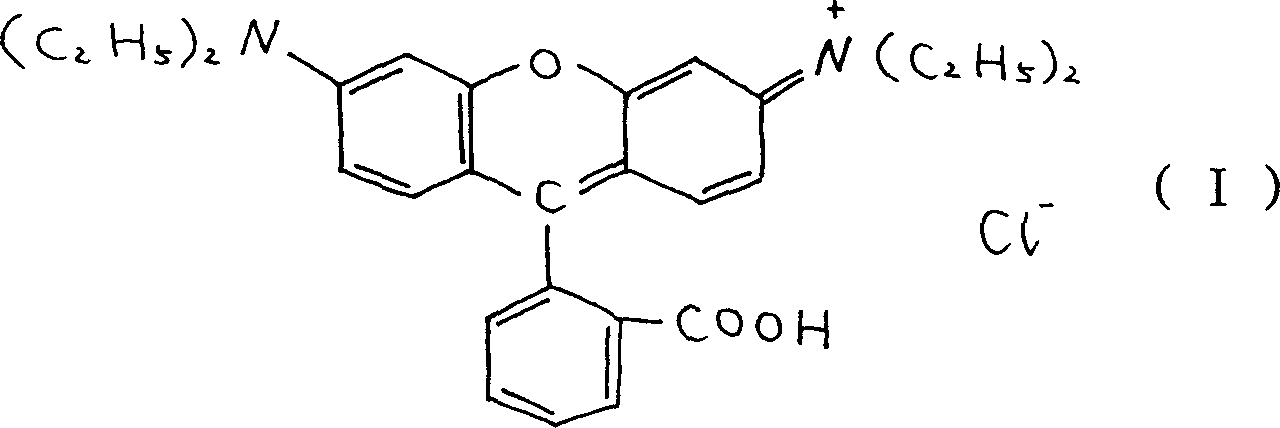
Prepn of rhodamin B
A technology of flask and m-hydroxy, which is applied in the field of dye preparation, can solve the problems of large water resources, high production cost, and long reaction time, and achieve the effects of reducing production cost, improving production efficiency, and shortening production time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] (1) At normal temperature, in the flask, drop into following raw material successively by following parts by weight: 145g o-dichlorobenzene, 48g anhydrous phthalic anhydride, 38g m-hydroxyl N, N-diethylaniline; Flask is set Dehydration separator and condenser, equipped with stirring device and thermometer;
[0023] (2) Start the stirring device, then feed nitrogen into the flask, and heat up to 175°C;
[0024] (3) Drop into the m-hydroxyl N, N-diethylaniline 5 times in the flask again, drop into 5g m-hydroxyl N, N-diethylaniline at every turn, promptly drop into the m-hydroxyl N of 25g altogether, N-diethylaniline Aniline;
[0025] (4) the flask was incubated at a temperature of 175° C. for 3 hours;
[0026] (5) Then the above-mentioned flask is cooled to 100°C, the reaction mixture in the flask is poured into a beaker, and the excess anhydrous phthalic anhydride in the reaction is neutralized with 300ml of 20% NaOH solution, and 400ml of 10% sulfuric acid is used for...
Embodiment 2
[0029] (1) At normal temperature, in the flask, drop into the following raw materials successively by the following parts by weight: 150g p-dichlorobenzene, 60g anhydrous phthalic anhydride, 38g m-hydroxyl N, N-diethylaniline; Dehydration separator and condenser, equipped with stirring device and thermometer;
[0030] (2) Start the stirring device, then feed nitrogen into the flask, and heat up to 175°C;
[0031] (3) Drop into the m-hydroxyl N, N-diethylaniline 5 times in the flask again, drop into 5g m-hydroxyl N, N-diethylaniline at every turn, promptly drop into the m-hydroxyl N of 25g altogether, N-diethylaniline (4) the flask was incubated at a temperature of 170°C for 4 hours; (5) the above-mentioned flask was then cooled to 100°C, the reaction mixture in the flask was poured into a beaker, and the reaction was neutralized with 400ml of 20% NaOH solution Excessive anhydrous phthalic anhydride, utilize 10% sulfuric acid 450ml to extract the material of the reaction mixtu...
Embodiment 3
[0034] (1) At normal temperature, in the flask, drop into the following raw materials successively by the following parts by weight: 130g p-dichlorobenzene, 40g anhydrous phthalic anhydride, 38g m-hydroxyl N, N-diethylaniline; Dehydration separator and condenser, equipped with stirring device and thermometer;
[0035] (2) Start the stirring device, then feed nitrogen into the flask, and heat up to 175°C;
[0036] (3) Drop into the m-hydroxyl N, N-diethylaniline 5 times in the flask again, drop into 5g m-hydroxyl N, N-diethylaniline at every turn, promptly drop into the m-hydroxyl N of 25g altogether, N-diethylaniline Aniline;
[0037] (4) the flask was incubated at a temperature of 170° C. for 4 hours;
[0038] (5) Then the above-mentioned flask is cooled to 100°C, the reaction mixture in the flask is poured into a beaker, and the excessive anhydrous phthalic anhydride in the neutralization reaction is neutralized with 300ml of 20% NaOH solution, and 400ml of 10% sulfuric ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com