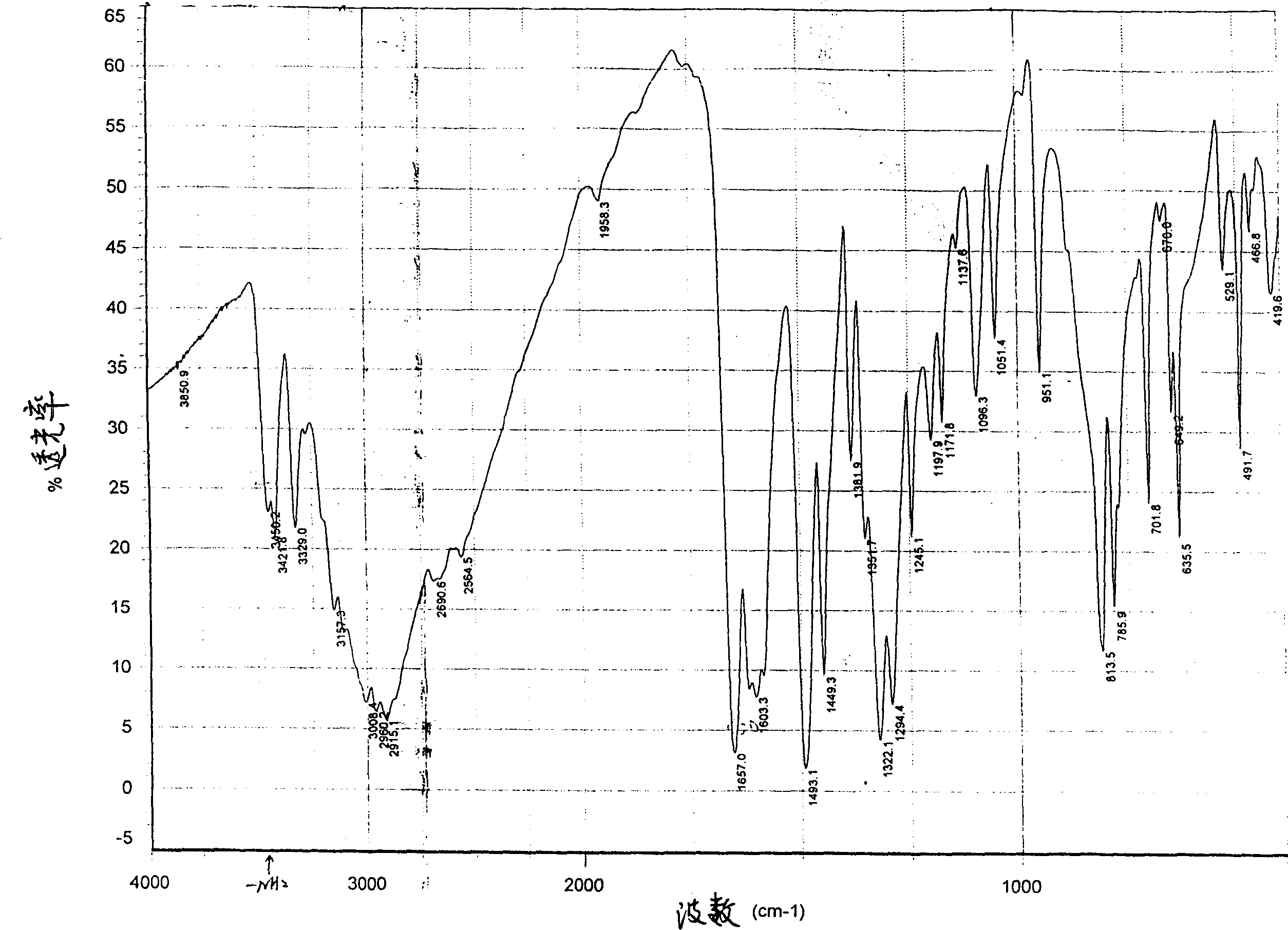
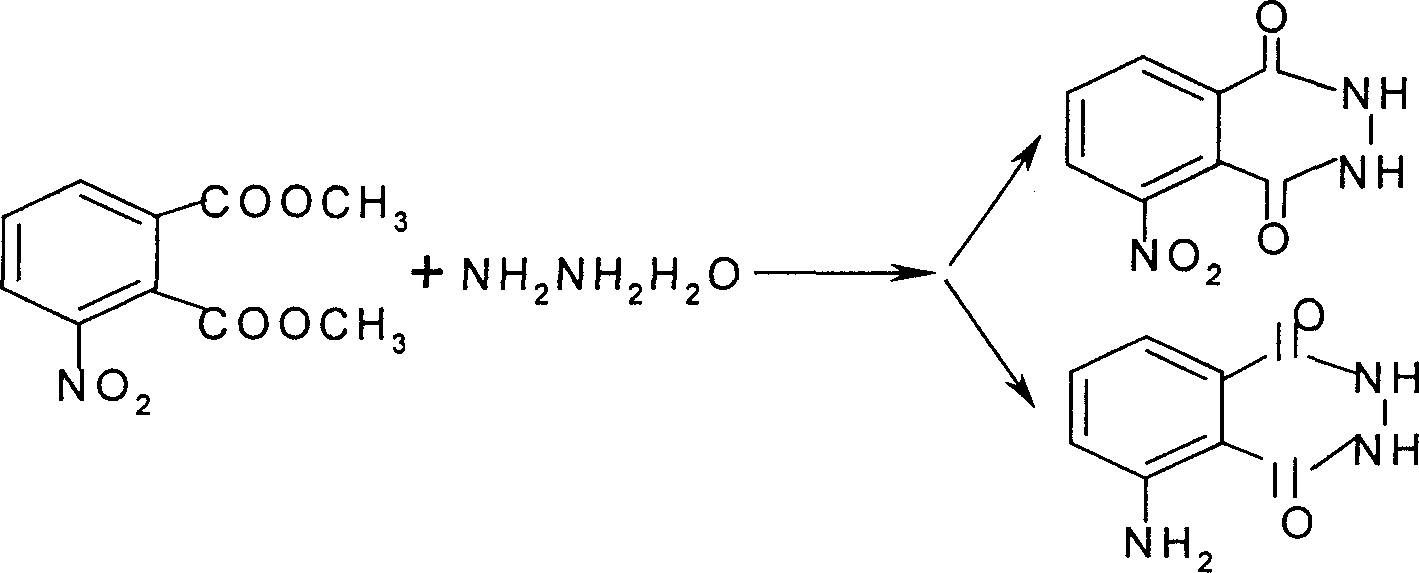
Method for synthesizing 3-nitro or 3-amino phthalyl hydrazine
A technology of nitrophthalic hydrazide and amino phthalic hydrazide is applied in the field of direct synthesis of 3-nitro phthalic hydrazide or 3-amino phthalic hydrazide, and can solve the problem of product Unstable quality, more waste water and other problems, to achieve the effect of simple operation, advanced and reasonable process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] The synthesis of embodiment 1.3-nitrophthalohydrazide
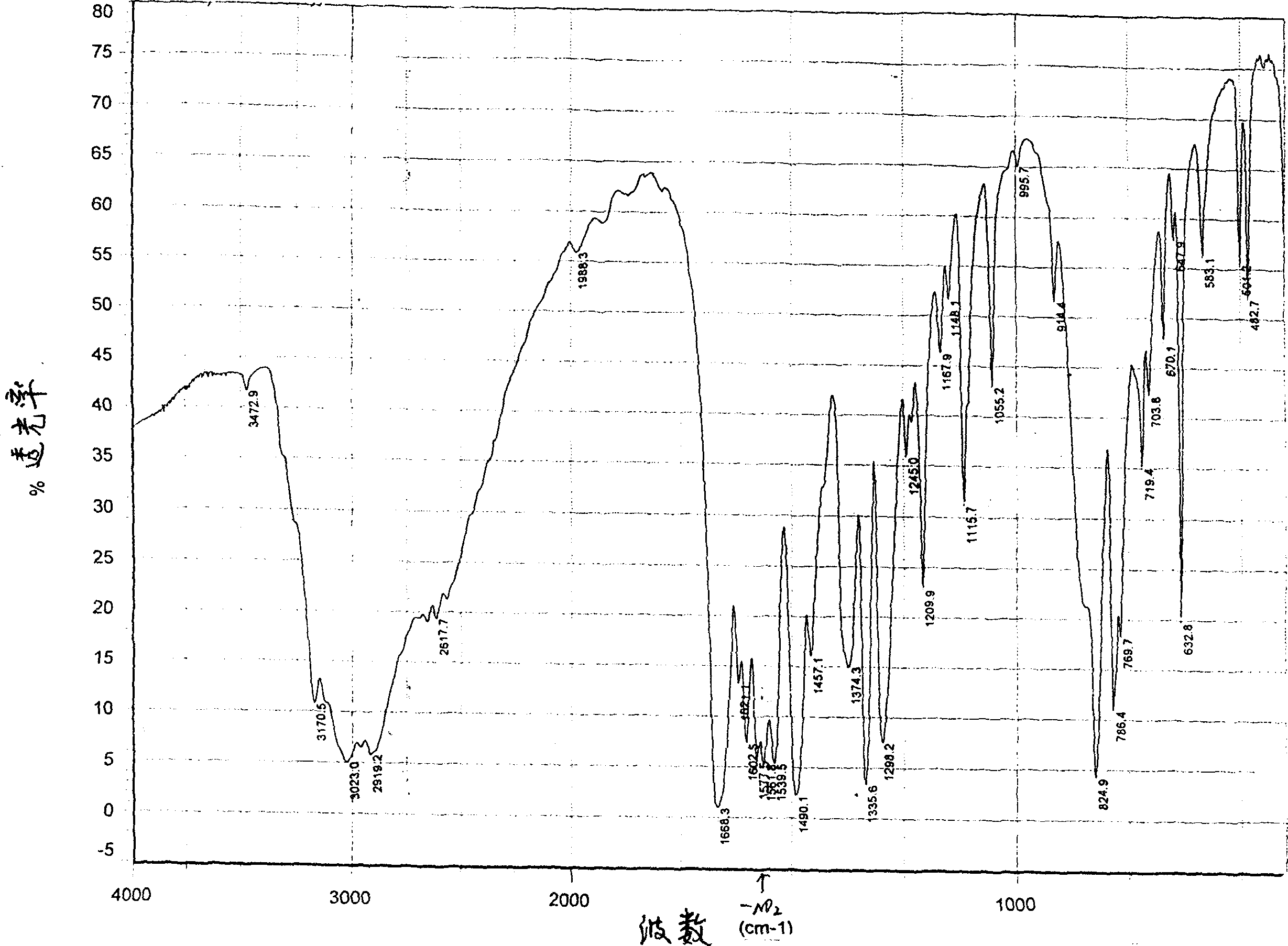
[0015] In a dry 1000ml three-neck flask, add 100g of dimethyl 3-nitrophthalate, 140g of ethanol, and 83.6g of hydrazine hydrate (50%), stir and heat to reflux, maintain the reflux reaction for 3h, stir to lower the temperature, and cool to Suction filter after room temperature. The crystals were dissolved in water and filtered, and the solution was acidified with concentrated hydrochloric acid to pH 1.5-2, filtered, washed with water, and dried to obtain 60 g. The appearance was light yellow crystalline powder, the yield was 69%, the purity was 98.2%, and the melting point was >300°C. Infrared spectrogram see figure 1 .
Embodiment 2
[0016] The synthesis of embodiment 2.3-nitrophthalohydrazide
[0017] Add 100g of dimethyl 3-nitrophthalate, 200g of methanol, and 63g of hydrazine hydrate (40%) into a dry 1000ml three-neck flask, stir and heat to reflux, maintain reflux for 2 hours, stir to lower the temperature, and cool to room temperature After suction filtration. The crystals were dissolved in water and filtered, and the solution was acidified with concentrated hydrochloric acid to pH 1.5-2, filtered, washed with water, and dried to obtain 58g. The appearance was light yellow crystalline powder, the yield was 67%, the purity was 98.2%, and the melting point was >300°C. Infrared spectrogram see figure 1 .
Embodiment 3
[0018] The synthesis of embodiment 3.3-nitrophthalohydrazide
[0019] Add 100g of dimethyl 3-nitrophthalate, 80g of ethanol, and 171g of hydrazine hydrate (60%) into a dry 1000ml three-neck flask, stir and heat to reflux, maintain reflux for 4 hours, stir to lower the temperature, and cool to room temperature After suction filtration. The crystals were dissolved in water and filtered, and the solution was acidified with concentrated hydrochloric acid to pH 1.5-2, filtered, washed with water, and dried to obtain 62 g of 3-nitrophthalhydrazide, which appeared as a light yellow crystalline powder with a yield of 72%. The purity is 98.2%, the melting point is >300°C, and its infrared spectrum is shown in figure 1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com