Lithium metal dispersion in electrodes
A technology of lithium metal and lithium metal powder, which is applied in the field of secondary batteries and can solve problems such as small capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Example 1 - Ethylene Propylene Diene Terpolymer and Cyclohexane
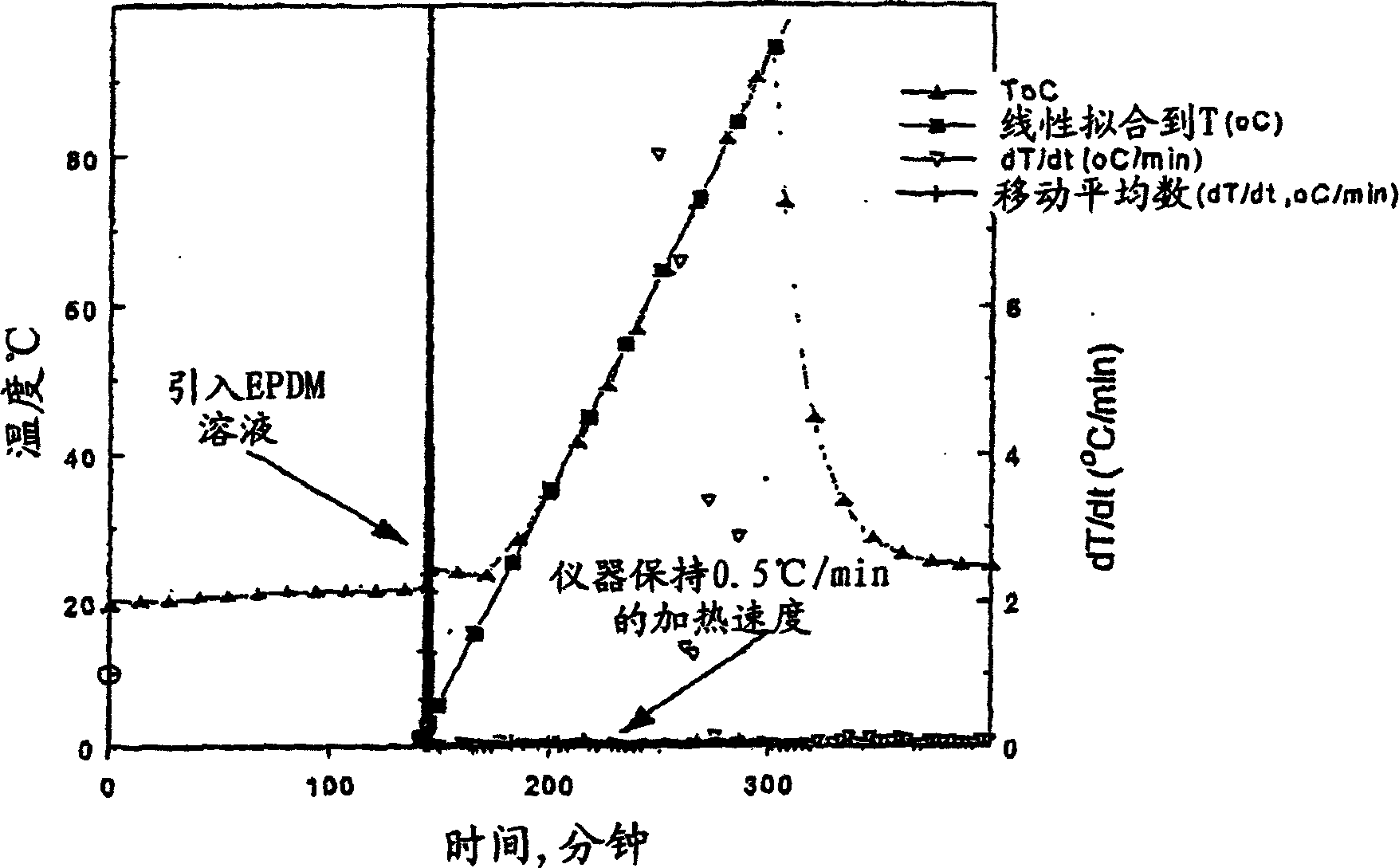
[0078] The test involved cyclohexane, lithium powder and ethylene propylene diene terpolymer (Nordel IP4570) thermal stability of the coating solution in the temperature range of interest. The solution consisted of 8.8 ml cyclohexane, 0.24 g lithium powder and 0.127 g ethylene propylene diene terpolymer. Use the reaction system screening tool as the calorimeter of choice. In the tests, the pressure inside the chamber was set at 200 psig using argon to allow the system to be tested beyond the boiling point of cyclohexane. No self-heating was detected in the temperature range of 19°C-94°C. The plot of the experiment is plotted in figure 2 middle. Cyclohexane has a boiling point of 80.7°C at 1 atm, so detection above this temperature is unnecessary, and the test was stopped at 94°C. Such as figure 2 As shown, during the ramp, the instrument maintains a steady-state heating rate of 0.5°C / min. If s...
Embodiment 2
[0079] Example 2 - Lithium powder and p-xylene
[0080] An amount of 0.531 g of lithium powder was mixed with 8 ml of p-xylene, and a thermal stability test was performed using the reaction system screening tool described in Example 1. Tests were performed between room temperature and 180°C. No self-heating was detected in this temperature range, implying that the lithium powder is stable in p-xylene between room temperature and 180 °C.
Embodiment 3- 2
[0081] Example 3 - Dimethylpropylene Urea and Lithium Powder
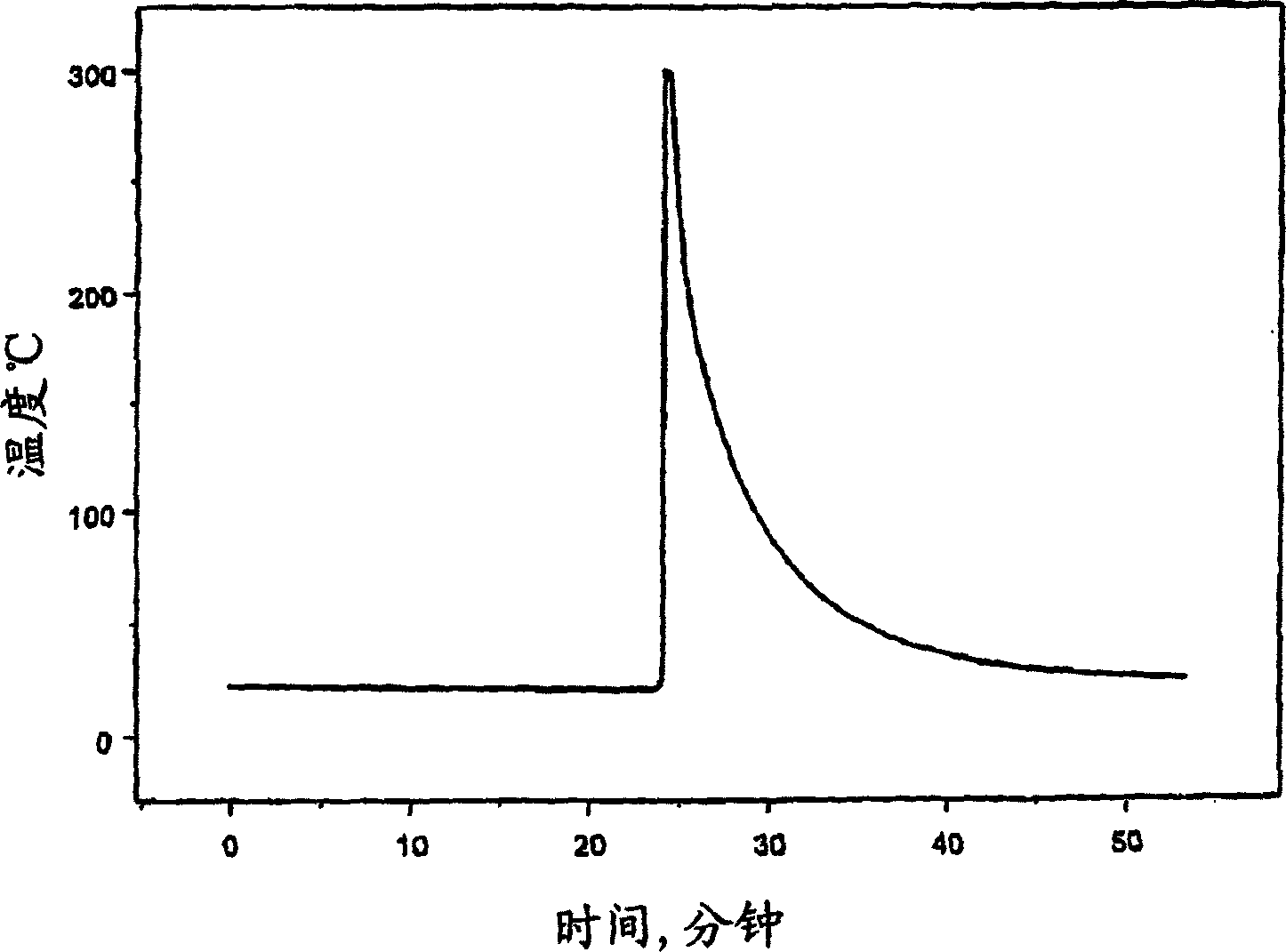
[0082] The thermal stability of the solution containing dimethylpropylene urea and lithium powder was tested using the same reaction system screening tool technique as described in Example 1 with the apparatus and procedure. Addition of dimethylpropylene urea to the lithium powder self-heating was detected within 3 seconds at a temperature of 25 °C. Self-heating increases at a rate of over 1000°C / min. image 3 The thermal runaway of the experiment is shown graphically. The presence of self-heating in the system suggests that dimethylpropylene urea is not a suitable solvent to form the anode of the present invention because it reacts with the lithium powder.
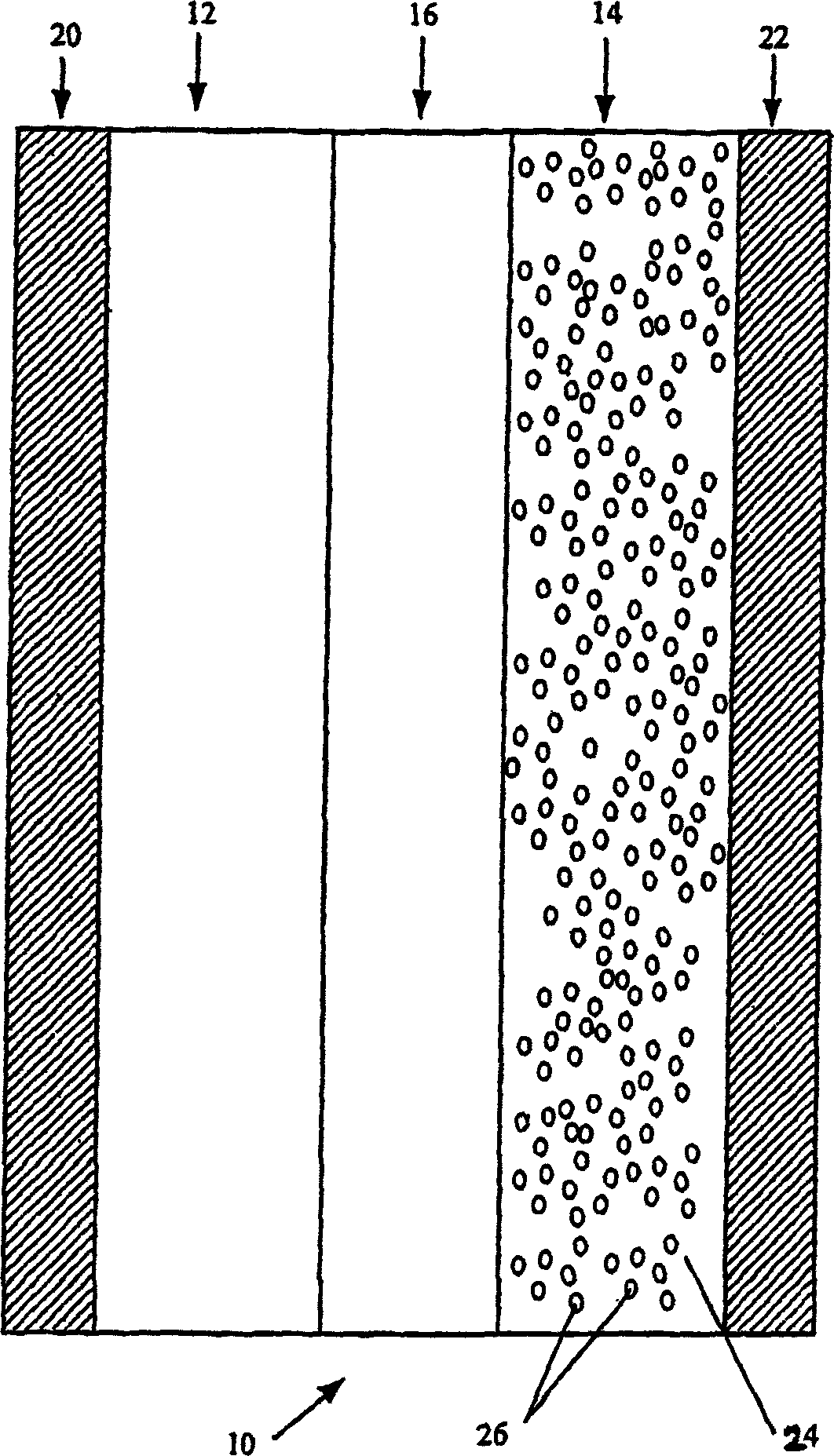
[0083] In another anode 14 production process, lithium metal may be provided in anode 14 by impregnating matrix material 24 in a suspension comprising lithium metal in a non-aqueous liquid such as a hydrocarbon solvent (eg, hexane). The lithium metal 26 use...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com