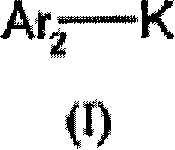Aryl and heteroaryl compounds, compositions, and methods of use
A technology for heteroaryl groups and compounds, which can be used in drug combinations, chemical instruments and methods, preparation of organic compounds, etc., and can solve problems such as no effective drug treatment.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0450] Scheme III describes the preparation of compounds of formula (4).
[0451] Ar 5 , Ar 6 are mutually independent groups such as, but not limited to, heteroaryl, heteroarylene, arylene, or aromatic ring systems.
[0452] As shown in Scheme III, in another embodiment, amino acid methyl esters (or other Esterified amino acids attached to Wang resin) (8) to form amides (9). The resulting amide is then coupled with an arylboronic acid or a heteroarylboronic acid in the presence of a catalyst such as but not limited to tetrakis(triphenylphosphine)palladium(0) and a base such as but not limited to sodium carbonate to form Compound (10). Hydrolysis of the methyl ester (10) with a base such as but not limited to LiOH yields the free carboxylic acid (4), where Ar 1 and Ar 2 The definition of the same formula (I).
[0453] Process III
[0454]
[0455] Scheme IV describes the preparation of compounds of formula (4).
[0456] Ar 3 , Ar 7 , Ar 5 and Ar 6 are mutually ...
Embodiment 1
[0688] (2S)-[5-Bromo-2-(4-trifluoromethylbenzyloxy)-benzamide]-3-(2′-phenoxybiphenyl-4-yl)-propionic acid
[0689] 5-Bromo-salicylic acid (2.16 g, 10 mmol) was first converted to 2-acetyl-5-bromo-salicylic acid ( 252g). The above acid (1.29 g, 5.0 mmol) was converted to the acid chloride with oxychloride (1.97 g, 15 mmol) and a catalytic amount of DMF in DCM, then 2-phenoxy-biphenylalanine was added to the acid chloride acid (1.45g, 5.0mmol) and DIEA (0.77g, 6.0mmol) to form (2S)-[5-bromo-2-hydroxybenzoylamine]-3-(2'-phenoxybiphenyl-4 -yl)-methyl propionate (1.92 g). Reaction of the above methyl ester (50 mg, 0.092 mmol) with 4-trifluoromethylbenzyl bromide (44 mg, 0.18 mmol) as described in General Procedure H affords (2S)-[5-bromo-2-(4- Trifluoromethylbenzyloxy)-benzoylamino]-3-(2'-phenoxybiphenyl-4-substituted)-propionic acid methyl ester (55 mg). The ester was hydrolyzed as in General Procedure C to give the title compound (52 mg).
[0690] 1 H-NMR (400MHz, CDCl 3 )...
Embodiment 2
[0692] (2S)-(5-Bromo-2-heptyloxy-benzoylamino)-3-[2′-(4-trifluoromethyl-phenoxy)-biphenyl-4-yl]-propionic acid
[0693] 5-Bromo-2-hydroxy-benzoic acid methyl ester (1.0 g, 4.32 mmol) was reacted with iodoheptane (1.46 g, 6.49 mmol) following general procedure H by addition of potassium carbonate (1.5 g, 10.8 mmol) to prepare 5- Bromo-2-heptyloxy-benzoic acid. The ester thus obtained was hydrolyzed following General Procedure C to give 5-bromo-2-heptyloxy-benzoic acid (0.950 g).
[0694] From 4-bromophenylalanine (5.g, 20.48mmol), 2-hydroxyphenylboronic acid (4.23g, 30.72mmol) and Pd (PPh 3 ) 4 (2.36g, 2.038mmol) according to process D to produce the corresponding amino acid, further esterified with anhydrous methanol containing 2-3ml HCl to produce the corresponding HCl salt (2S)-amino-3-(2'-hydroxy-biphenyl -4-yl)-propionic acid methyl ester (5.0 g) to prepare (2S)-amino-3-(2'-hydroxybiphenyl-4-yl)-propionic acid.
[0695] 5-Bromo-2-heptyloxy-benzoic acid (0.231 g, 0.738 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com