Motherwort wort drip pills and prepn. method
A technology of motherwort and grass dripping pills, which is applied in the direction of pill delivery, pharmaceutical formulations, and medical preparations containing active ingredients, etc., can solve the problems of hepatic and intestinal first-pass effect bioavailability, affect the therapeutic effect, and low bioavailability, and achieve Avoid the first-pass effect, the process is reasonable, and the effect of high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
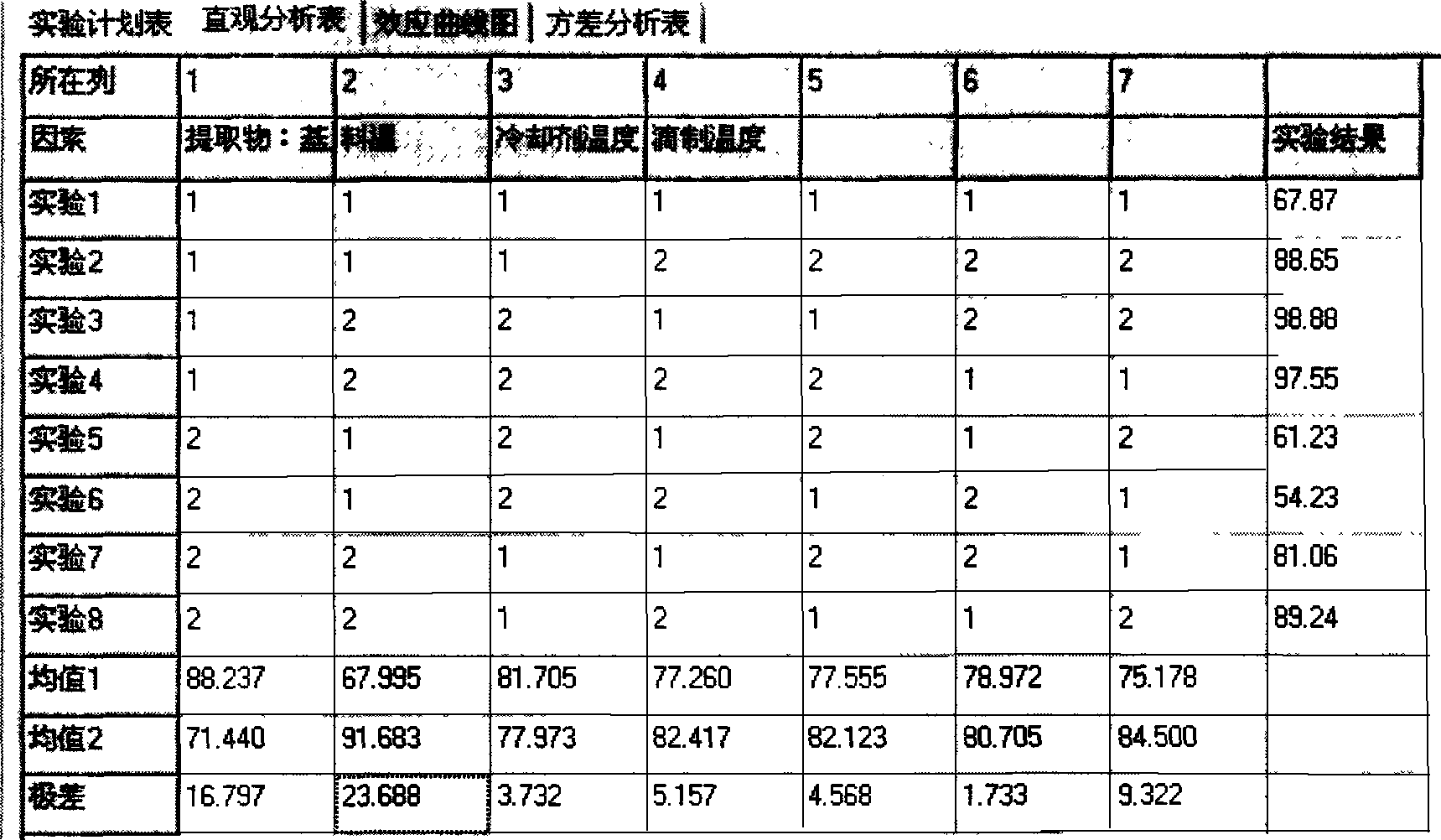
[0039] Example 1: drug and matrix ratio test
[0040] Fix the drop distance (about 6cm), the length of the condensation column (80cm), the gradient cooling liquid (the temperature of the upper part of the temperature is 30±2°C, the temperature of the lower part of the nozzle is 10±2°C), the temperature of the oil bath is 100±5°C, and then Carry out combination test, take the smooth roundness of dropping pill as the investigation index, the results are shown in Table 1
[0041] Polyethylene glycol-4000
polyethylene glycol 1500
Polyethylene glycol-6000
matrix with
drug ratio
drop system
temperature °C
smooth round
Integer rate (%)
matrix with
drug ratio
drop system
temperature °C
smooth round
Integer rate (%)
matrix with
drug ratio
drop system
temperature °C
smooth round
Integer rate (%)
1.2∶1
1.2∶1
1.6∶1
1.6∶1
2.0∶1
2.0∶1
95±2
110±2
95±2...
example 2
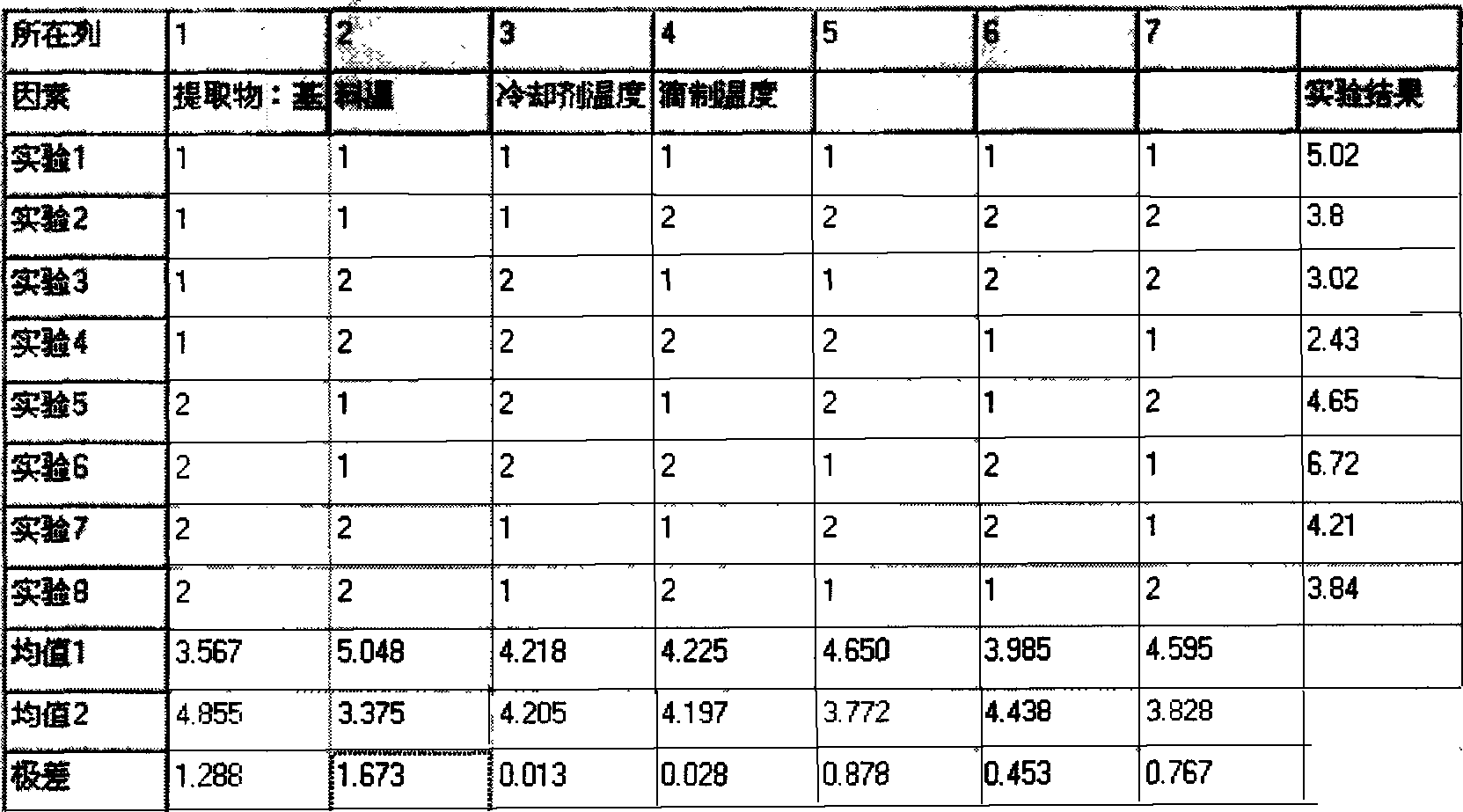
[0044] Example 2: The way to add the drug to the matrix is selected
[0045] Fixed drop distance (about 6cm), condensation column length (80cm), gradient cooling liquid (temperature at the upper part is 30+2℃, temperature at the lower part of the nozzle is 10±2℃), oil bath temperature is 100±5℃, substrate and The ratio of the drug was 8:5, the dropping temperature was 95±2°C, and the drug was added to the molten matrix in stages. The test results are shown in Table 2.
[0046] Feeding method
[0047] After the drug is added at one time, due to the large amount of drug, it is difficult to mix evenly. The appearance of the dropping pill is often rough, and the formability is not good. When the drug is added to the molten matrix in stages, it can be fully stirred. The smooth rounding rate is also high.
example 3
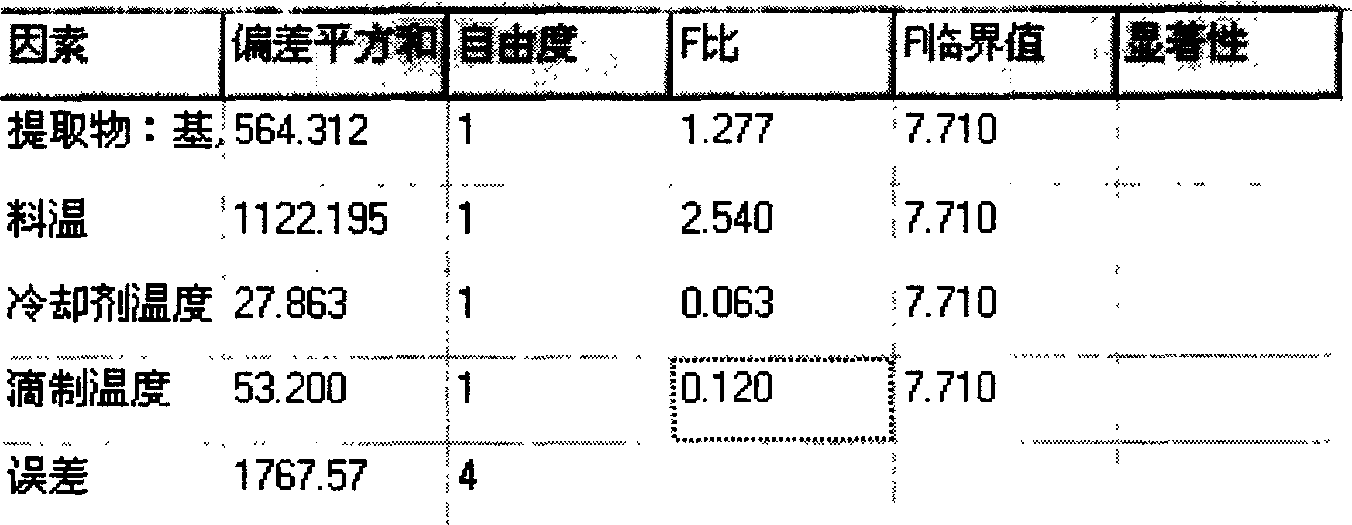
[0048] Example 3: Experiments with different dripper temperatures
[0049] The drug was added to the molten Matrix comparison, the test results are shown in Table 3.
[0050] Substrate: drug
[0051] When the dropping pills enter the condensate, the temperature of the coolant (20cm) is too low (20±2°C), and the prepared dropping pills often have voids or irregular pill shapes; when the temperature is too high (45±2°C), The density of the coolant here is low, the sinking speed of the prepared drop pills is too fast, and cannot be fully cooled, and flat pills or adhesions often occur; when the temperature of the coolant is controlled to 30±2°C, the roundness of the obtained drop pills is relatively high , The pill shape is smooth, therefore, the coolant should be controlled at 30±2°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com